World J Mens Health.
2017 Aug;35(2):77-93. 10.5534/wjmh.2017.35.2.77.
Role of Antioxidants in Assisted Reproductive Techniques
- Affiliations
-
- 1American Center for Reproductive Medicine and the Department of Urology, Cleveland Clinic, Cleveland, OH, USA. agarwaa@ccf.org
- 2Department of Urology, Hamad Medical Hospital, Doha, Qatar.
- KMID: 2388443
- DOI: http://doi.org/10.5534/wjmh.2017.35.2.77
Abstract
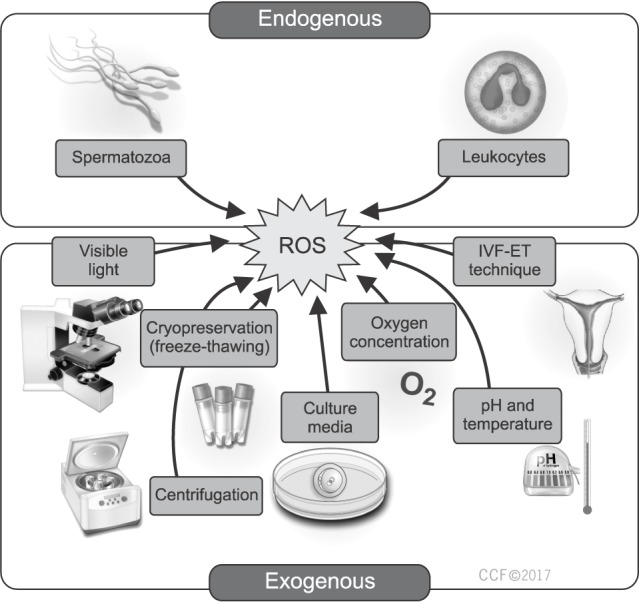
- Oxidative stress (OS) has been recognized as a significant cause of suboptimal assisted reproductive outcome. Many of the sperm preparation and manipulation procedures that are necessary in the in vitro environment can result in excessive production of reactive oxygen species (ROS) thereby exposing the gametes and growing embryos to significant oxidative damage. Antioxidants have long been utilized in the management of male subfertility as they can counterbalance the elevated levels of ROS inducing a high state of OS. Few studies have looked into the clinical effectiveness of antioxidants in patients undergoing assisted reproduction. While an overall favorable outcome has been perceived, the specific clinical indication and optimal antioxidant regimen remain unknown. The goal of our review is to explore the sources of ROS in the in vitro environment and provide a clinical scenario-based approach to identify the circumstances where antioxidant supplementation is most beneficial to enhance the outcome of assisted reproduction.
MeSH Terms
Figure
Reference
-
1. Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002; 77:873–882. PMID: 12009338.
Article2. Zhu T. In vitro fertilization [Internet]. Arizona: Embryo Project Encyclopedia;c2009. cited 2017 Mar 14. Available from: http://embryo.asu.edu/handle/10776/1665.3. Brezina PR, Zhao Y. The ethical, legal, and social issues impacted by modern assisted reproductive technologies. Obstet Gynecol Int. 2012; 2012:686253. PMID: 22272208.
Article4. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79:829–843. PMID: 12749418.
Article5. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014; 32:1–17. PMID: 24872947.
Article6. Zini A, Al-Hathal N. Antioxidant therapy in male infertility: fact or fiction? Asian J Androl. 2011; 13:374–381. PMID: 21516118.
Article7. Agarwal A, Majzoub A. Role of antioxidants in male infertility. BJUI. 2016; DOI: 10.18591/BJUIK.0510.
Article8. Halliwell B. Free radicals and vascular disease: how much do we know? BMJ. 1993; 307:885–886. PMID: 8241848.
Article9. de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997; 2:48–54. PMID: 9414465.
Article10. Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995; 108:2017–2025. PMID: 7544800.
Article11. de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995; 10(Suppl 1):15–21. PMID: 8592032.
Article12. Zini A, De Lamirande E, Gagnon C. Low levels of nitric oxide promote human sperm capacitation in vitro. J Androl. 1995; 16:424–431. PMID: 8575982.13. Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993; 215:213–219. PMID: 7688300.
Article14. Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006; 86:503–512. PMID: 16860798.
Article15. du Plessis SS, Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Expet Rev Obstet Gynecol. 2008; 3:539–554.
Article16. Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008; 59:2–11. PMID: 18154591.17. Agarwal A, Majzoub A. Free radicals in andrology. In : Balercia G, Gandini L, Lenzi A, Lombardo F, editors. Antioxidants in andrology. Cham: Springer International Publishing;2017. p. 1–21.18. Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001; 16:1922–1930. PMID: 11527899.
Article19. Tanphaichitr N, Kongmanas K, Kruevaisayawan H, Saewu A, Sugeng C, Fernandes J, et al. Remodeling of the plasma membrane in preparation for sperm-egg recognition: roles of acrosomal proteins. Asian J Androl. 2015; 17:574–582. PMID: 25994642.
Article20. Aitken RJ, Baker MA. Reactive oxygen species generation by human spermatozoa: a continuing enigma. Int J Androl. 2002; 25:191–194. PMID: 12121567.
Article21. Schatten H, Sun QY, Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol. 2014; 12:111. PMID: 25421171.
Article22. Sandoval JS, Raburn D, Mausher S. Leukocytospermia: overview of diagnosis, implications, and management of a controversial finding. Middle East Fertil Soc J. 2013; 18:129–134.
Article23. Henkel R, Schill WB. Sperm separation in patients with urogenital infections. Andrologia. 1998; 30(Suppl 1):91–97.
Article24. Lobascio AM, De Felici M, Anibaldi M, Greco P, Minasi MG, Greco E. Involvement of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology. 2015; 3:265–270. PMID: 25598385.
Article25. Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005; 83:635–642. PMID: 15749492.
Article26. Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002; 78:1215–1224. PMID: 12477515.
Article27. Mupfiga C, Fisher D, Kruger T, Henkel R. The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst Biol Reprod Med. 2013; 59:304–311. PMID: 23898825.
Article28. Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. 2002; 77:1184–1190. PMID: 12057726.
Article29. Lampiao F. Free radicals generation in an in vitro fertilization setting and how to minimize them. World J Obstet Gynecol. 2012; 1:29–34.30. Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, et al. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril. 2004; 82:593–600. PMID: 15374701.
Article31. Rakhit M, Gokul SR, Agarwal A, du Plessis SS. Antioxidant strategies to overcome OS in IVF-embryo transfer. In : Agarwal A, Azia N, Rizk B, editors. Studies on women's health. New York: Humana Press;2013. p. 237–262.32. Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003; 9:331–345. PMID: 12926527.
Article33. Gandini L, Lombardo F, Paoli D, Caponecchia L, Familiari G, Verlengia C, et al. Study of apoptotic DNA fragmentation in human spermatozoa. Hum Reprod. 2000; 15:830–839. PMID: 10739828.
Article34. Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002; 17:426–431. PMID: 11821289.
Article35. Bavister B. Oxygen concentration and preimplantation development. Reprod Biomed Online. 2004; 9:484–486. PMID: 15588462.
Article36. Kasterstein E, Strassburger D, Komarovsky D, Bern O, Komsky A, Raziel A, et al. The effect of two distinct levels of oxygen concentration on embryo development in a sibling oocyte study. J Assist Reprod Genet. 2013; 30:1073–1079. PMID: 23835722.
Article37. Beehler BC, Przybyszewski J, Box HB, Kulesz-Martin MF. Formation of 8-hydroxydeoxyguanosine within DNA of mouse keratinocytes exposed in culture to UVB and H2O2. Carcinogenesis. 1992; 13:2003–2007. PMID: 1423868.
Article38. Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci U S A. 2007; 104:14289–14293. PMID: 17709739.
Article39. Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B. 2001; 63:103–113. PMID: 11684457.
Article40. Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the preimplantation embryo and its surroundings. Hum Reprod Update. 2001; 7:175–189. PMID: 11284661.41. Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000; 149:43–50. PMID: 10963860.
Article42. Nasr-Esfahani MH, Johnson MH. How does transferrin overcome the in vitro block to development of the mouse preimplantation embryo? J Reprod Fertil. 1992; 96:41–48. PMID: 1432973.
Article43. Will MA, Clark NA, Swain JE. Biological pH buffers in IVF: help or hindrance to success. J Assist Reprod Genet. 2011; 28:711–724. PMID: 21614519.
Article44. Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant. 2006; 126:45–51.
Article45. Ferguson WJ, Braunschweiger KI, Braunschweiger WR, Smith JR, McCormick JJ, Wasmann CC, et al. Hydrogen ion buffers for biological research. Anal Biochem. 1980; 104:300–310. PMID: 7446957.
Article46. Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002; 128:682–695. PMID: 11842171.
Article47. Shekarriz M, DeWire DM, Thomas AJ Jr, Agarwal A. A method of human semen centrifugation to minimize the iatrogenic sperm injuries caused by reactive oxygen species. Eur Urol. 1995; 28:31–35. PMID: 8521891.48. Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003; 1:108. PMID: 14617368.49. Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2015; 1257:3–19. PMID: 25428001.
Article50. Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol. 2012; 2012:854837. PMID: 22194740.
Article51. Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl. 1992; 13:232–241. PMID: 1601742.52. Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009; 24:2061–2070. PMID: 19525298.
Article53. Zribi N, Feki Chakroun N, El Euch H, Gargouri J, Bahloul A, Ammar Keskes L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril. 2010; 93:159–166. PMID: 19027111.
Article54. Hammadeh ME, Al Hasani S, Rosenbaum P, Schmidt W, Fischer Hammadeh C. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch Gynecol Obstet. 2008; 277:515–526. PMID: 18026972.
Article55. Ozatik O, Aydin Y, Hassa H, Ulusoy D, Ogut S, Sahin F. Relationship between oxidative stress and clinical pregnancy in assisted reproductive technology treatment cycles. J Assist Reprod Genet. 2013; 30:765–772. PMID: 23666546.
Article56. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010; 16:231–245. PMID: 19934213.
Article57. Foote RH, Brockett CC, Kaproth MT. Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim Reprod Sci. 2002; 71:13–23. PMID: 11988368.
Article58. Zribi N, Chakroun NF, Ben Abdallah F, Elleuch H, Sellami A, Gargouri J, et al. Effect of freezing-thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology. 2012; 65:326–331. PMID: 23010483.
Article59. Breininger E, Beorlegui NB, O'Flaherty CM, Beconi MT. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology. 2005; 63:2126–2135. PMID: 15826678.
Article60. Moubasher AE, El Din AM, Ali ME, El-sherif WT, Gaber HD. Catalase improves motility, vitality and DNA integrity of cryopreserved human spermatozoa. Andrologia. 2013; 45:135–139. PMID: 22591546.
Article61. Amália PM, Possa MN, Augusto MC, Francisca LS. Quercetin prevents oxidative stress in cirrhotic rats. Dig Dis Sci. 2007; 52:2616–2621. PMID: 17431769.
Article62. Shaik YB, Castellani ML, Perrella A, Conti F, Salini V, Tete S, et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006; 20:47–52. PMID: 18187018.63. Gibb Z, Butler TJ, Morris LH, Maxwell WM, Grupen CG. Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology. 2013; 79:1001–1009. PMID: 23453253.
Article64. Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004; 61:192–208. PMID: 14745498.
Article65. Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004; 44:275–295. PMID: 15462130.
Article66. Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology. 2010; 60:235–237. PMID: 19895799.
Article67. Garcez ME, dos Santos Branco C, Lara LV, Pasqualotto FF, Salvador M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil Steril. 2010; 94:2118–2121. PMID: 20189559.
Article68. Bucak MN, Ataman MB, Başpınar N, Uysal O, Taşpınar M, Bilgili A, et al. Lycopene and resveratrol improve post-thaw bull sperm parameters: sperm motility, mitochondrial activity and DNA integrity. Andrologia. 2015; 47:545–552. PMID: 24909239.
Article69. Silva EC, Cajueiro JF, Silva SV, Soares PC, Guerra MM. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology. 2012; 77:1722–1726. PMID: 22289215.
Article70. Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK. Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril. 2011; 95:1149–1151. PMID: 21067726.
Article71. Taylor K, Roberts P, Sanders K, Burton P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod Biomed Online. 2009; 18:184–189. PMID: 19192337.
Article72. Noack-Füller G, De Beer C, Seibert H. Cadmium, lead, selenium, and zinc in semen of occupationally unexposed men. Andrologia. 1993; 25:7–12. PMID: 8427423.
Article73. Siegel RB, Murray FA, Julien WE, Moxon AL, Conrad HR. Effect of in vitro selenium supplementation on bovine sperm motility. Theriogenology. 1980; 13:357–367. PMID: 16725505.74. Dorostkar K, Alavi-Shoushtari SM, Mokarizadeh A. Effects of in vitro selenium addition to the semen extender on the spermatozoa characteristics before and after freezing in water buffaloes (Bubalus bubalis). Vet Res Forum. 2012; 3:263–268. PMID: 25653769.75. Geva E, Bartoov B, Zabludovsky N, Lessing JB, Lerner-Geva L, Amit A. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril. 1996; 66:430–434. PMID: 8751743.
Article76. Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996; 17:530–537. PMID: 8957697.77. Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen. 2002; 22:227–234. PMID: 11948633.
Article78. Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988; 9:367–376. PMID: 3215823.
Article79. Jacob RA, Pianalto FS, Agee RE. Cellular ascorbate depletion in healthy men. J Nutr. 1992; 122:1111–1118. PMID: 1564563.
Article80. Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991; 88:11003–11006. PMID: 1763015.
Article81. Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013; 27:325–337. PMID: 23948450.
Article82. Dawson EB, Harris WA, Teter MC, Powell LC. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril. 1992; 58:1034–1039. PMID: 1426355.83. Thiele JJ, Friesleben HJ, Fuchs J, Ochsendorf FR. Ascorbic acid and urate in human seminal plasma: determination and interrelationships with chemiluminescence in washed semen. Hum Reprod. 1995; 10:110–115. PMID: 7745037.
Article84. Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005; 26:349–353. PMID: 15867002.85. Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007; 47:216–221. PMID: 17550489.
Article86. Verma A, Kanwar KC. Human sperm motility and lipid peroxidation in different ascorbic acid concentrations: an in vitro analysis. Andrologia. 1998; 30:325–329. PMID: 9835946.
Article87. Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003; 80(Suppl 2):844–850. PMID: 14505763.
Article88. Mongioi L, Calogero AE, Vicari E, Condorelli RA, Russo GI, Privitera S, et al. The role of carnitine in male infertility. Andrology. 2016; 4:800–807. PMID: 27152678.
Article89. Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, et al. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004; 81:1578–1584. PMID: 15193480.
Article90. Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005; 84:662–671. PMID: 16169400.
Article91. Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl. 2004; 25:761–770. discussion 771-2. PMID: 15292108.92. Moslemi Mehni N, Ketabchi AA, Hosseini E. Combination effect of Pentoxifylline and L-carnitine on idiopathic oligo-asthenoteratozoospermia. Iran J Reprod Med. 2014; 12:817–824. PMID: 25709639.93. Al-Dujaily SS, Al-Sultani YK, Shams Alddin NN. DNA normality following in vitro sperm preparation with pentoxifylline and L-Carnitine for asthenozoospermic infertile men. Glob J Med Res. 2013; 13:25–30.94. Lewin A, Lavon H. The effect of coenzyme Q10 on sperm motility and function. Mol Aspects Med. 1997; 18(Suppl):S213–S219. PMID: 9266524.
Article95. Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014; 46:177–183. PMID: 23289958.
Article96. Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009; 182:237–248. PMID: 19447425.
Article97. Safarinejad MR. The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: an open-label prospective study. Int Urol Nephrol. 2012; 44:689–700. PMID: 22081410.
Article98. Giacone F, Condorelli RA, Mongioì LM, Bullara V, La Vignera S, Calogero AE. In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine. 2016; DOI: 10.1007/s12020-016-1013-7. [Epub].
Article99. Mora-Esteves C, Shin D. Nutrient supplementation: improving male fertility fourfold. Semin Reprod Med. 2013; 31:293–300. PMID: 23775385.
Article100. Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009; 19:638–659. PMID: 20021713.
Article101. Lenzi A, Picardo M, Gandini L, Lombardo F, Terminali O, Passi S, et al. Glutathione treatment of dyspermia: effect on the lipoperoxidation process. Hum Reprod. 1994; 9:2044–2050. PMID: 7868672.102. Oeda T, Henkel R, Ohmori H, Schill WB. Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility? Andrologia. 1997; 29:125–131. PMID: 9197915.
Article103. Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009; 74:73–76. PMID: 19428083.
Article104. Aitken RJ, Gibb Z, Mitchell LA, Lambourne SR, Connaughton HS, De Iuliis GN. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod. 2012; 87:110. PMID: 22933515.
Article105. Erkkilä K, Hirvonen V, Wuokko E, Parvinen M, Dunkel L. N-acetyl-L-cysteine inhibits apoptosis in human male germ cells in vitro. J Clin Endocrinol Metab. 1998; 83:2523–2531. PMID: 9661638.106. Castagné V, Lefèvre K, Natero R, Clarke PG, Bedker DA. An optimal redox status for the survival of axotomized ganglion cells in the developing retina. Neuroscience. 1999; 93:313–320. PMID: 10430495.
Article107. Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011; 13:43–52. PMID: 21076433.
Article108. Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016; 5:935–950. PMID: 28078226.
Article109. López G, Lafuente R, Checa MA, Carreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl. 2013; 15:790–794. PMID: 23912311.
Article110. Luna D, Hilario R, Dueñas-Chacón J, Romero R, Zavala P, Villegas L, et al. The IMSI procedure improves laboratory and clinical outcomes without compromising the aneuploidy rate when compared to the classical ICSI procedure. Clin Med Insights Reprod Health. 2015; 9:29–37. PMID: 26609251.
Article111. Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997; 68:519–524. PMID: 9314926.
Article112. Greco E, Romano S, Iacobelli M, Ferrero S, Baroni E, Minasi MG, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod. 2005; 20:2590–2594. PMID: 15932912.
Article113. Abad C, Amengual MJ, Gosálvez J, Coward K, Hannaoui N, Benet J, et al. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia. 2013; 45:211–216. PMID: 22943406.
Article114. Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod. 1998; 13:1240–1247. PMID: 9647554.
Article115. Boxmeer JC, Smit M, Utomo E, Romijn JC, Eijkemans MJ, Lindemans J, et al. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertil Steril. 2009; 92:548–556. PMID: 18722602.
Article116. Swayne BG, Kawata A, Behan NA, Williams A, Wade MG, Macfarlane AJ, et al. Investigating the effects of dietary folic acid on sperm count, DNA damage and mutation in Balb/c mice. Mutat Res. 2012; 737:1–7. PMID: 22824165.
Article117. da Silva TM, Silva Maia MC, Arruda JT, Approbato FC, Mendonça RC, Approbato MS. Folic acid does not improve semen parametrs in subfertile men: a double-blin, randomized, placebo-controlled study. JBRA Assist Reprod. 2013; 17:152–157.
Article118. Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, et al. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia. 2014; 46:956–962. PMID: 24147895.
Article119. Lee VM, Wong JS, Loh SK, Leong NK. Sperm motility in the semen analysis affects the outcome of superovulation intrauterine insemination in the treatment of infertile Asian couples with male factor infertility. BJOG. 2002; 109:115–120. PMID: 11905427.
Article120. Attallah D, El-Nashar IH, Mahmoud R, Shaaban OM, Salman SA. N-acytelcysteine prior to intrauterine insemination in couples with isolated athenozospermia: a randomized controlled trial. Fertil Steril. 2013; 100:S462.
Article121. Peivandi S, Karimpour A, Moslemizadeh N. Effects of L-carnitine on infertile men's spermogram; a randomized clinical trial. J Reprod Infertil. 2010; 10:245–251.122. Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005; 20:1006–1012. PMID: 15665024.
Article123. Fernandez CD, Porto EM, Arena AC, Kempinas Wde G. Effects of altered epididymal sperm transit time on sperm quality. Int J Androl. 2008; 31:427–437. PMID: 17822422.
Article124. Wood S, Sephton V, Searle T, Thomas K, Schnauffer K, Troup S, et al. Effect on clinical outcome of the interval between collection of epididymal and testicular spermatozoa and intracytoplasmic sperm injection in obstructive azoospermia. J Androl. 2003; 24:67–72. PMID: 12514085.125. Verheyen G, Joris H, Crits K, Nagy Z, Tournaye H, Van Steirteghem A. Comparison of different hypo-osmotic swelling solutions to select viable immotile spermatozoa for potential use in intracytoplasmic sperm injection. Hum Reprod Update. 1997; 3:195–203. PMID: 9322097.
Article126. Dalzell LH, McVicar CM, McClure N, Lutton D, Lewis SE. Effects of short and long incubations on DNA fragmentation of testicular sperm. Fertil Steril. 2004; 82:1443–1445. PMID: 15533376.
Article127. Nabi A, Khalili MA, Halvaei I, Roodbari F. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia. 2014; 46:374–379. PMID: 24689689.
Article128. Dalzell LH, Thompson-Cree ME, McClure N, Traub AI, Lewis SE. Effects of 24-hour incubation after freeze-thawing on DNA fragmentation of testicular sperm from infertile and fertile men. Fertil Steril. 2003; 79(Suppl 3):1670–1672. PMID: 12801581.
Article129. Salhiyyah K, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2012; 1:CD005262. PMID: 22258961.
Article130. Aparicio NJ, de Turner EA, Schwarzstein L, Turner D. Effect of the phosphodiesterase inhibitor Pentoxyfylline on human sperm motility. Andrologia. 1980; 12:49–54. PMID: 7377553.
Article131. Tournaye H, Janssens R, Devroey P, van Steirteghem A. The influence of pentoxifylline on motility and viability of spermatozoa from normozoospermic semen samples. Int J Androl. 1994; 17:1–8. PMID: 8005702.
Article132. Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl. 2006; 27:45–52. PMID: 16400077.
Article133. Griveau JF, Lobel B, Laurent MC, Michardière L, Le Lannou D. Interest of pentoxifylline in ICSI with frozen-thawed testicular spermatozoa from patients with non-obstructive azoospermia. Reprod Biomed Online. 2006; 12:14–18. PMID: 16454927.
Article134. Taşdemir I, Taşdemir M, Tavukçuoğlu S. Effect of pentoxifylline on immotile testicular spermatozoa. J Assist Reprod Genet. 1998; 15:90–92. PMID: 9513848.
Article135. Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, et al. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000; 17:194–199. PMID: 10955242.136. Wendel A. Measurement of in vivo lipid peroxidation and toxicological significance. Free Radic Biol Med. 1987; 3:355–358. PMID: 3319801.
Article137. Ufer C, Wang CC, Borchert A, Heydeck D, Kuhn H. Redox control in mammalian embryo development. Antioxid Redox Signal. 2010; 13:833–875. PMID: 20367257.
Article138. Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther. 2012; 342:608–618. PMID: 22700427.
Article139. Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013; 288:191–199. PMID: 23543240.
Article140. Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007; 14:418–421. PMID: 17425820.
Article141. Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. Is ascorbate able to reduce disulfide bridges? A cautionary note. Nitric Oxide. 2008; 19:252–258. PMID: 18675931.
Article142. Agarwal A, Wang SM. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology. 2017; DOI: 10.1016/j.urology.2017.02.016. [Epub].
Article143. Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017; 34:48–57. PMID: 27839743.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of assisted reproductive technology in Korea, 2007
- Current status of assisted reproductive technology in Korea, 2008
- Current Status of Assisted Reproductive Technology in Korea, 1999
- Current Status of Assisted Reproductive Technology in Korea, 2000
- Current Status of Assisted Reproductive Technology in Korea, 2002