Immune Netw.
2017 Aug;17(4):214-227. 10.4110/in.2017.17.4.214.
Cytokine Signaling in Tumor Progression
- Affiliations
-
- 1Department of Bioscience and Biotechnology, Sejong University, Seoul 05006, Korea. nature@sejong.ac.kr
- KMID: 2388076
- DOI: http://doi.org/10.4110/in.2017.17.4.214
Abstract
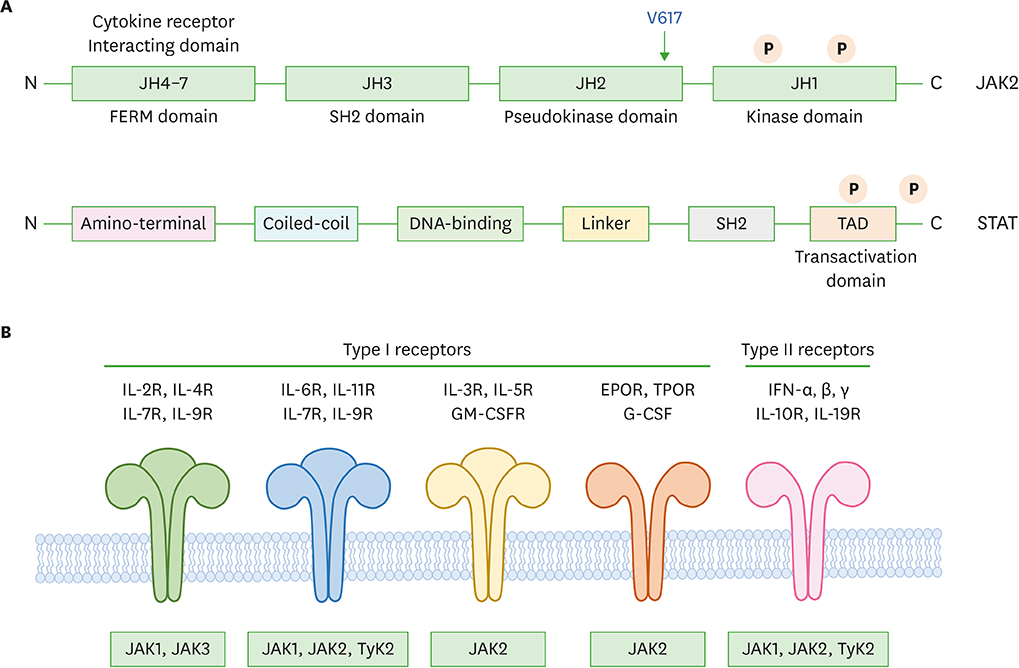
- Cytokines are molecules that play critical roles in the regulation of a wide range of normal functions leading to cellular proliferation, differentiation and survival, as well as in specialized cellular functions enabling host resistance to pathogens. Cytokines released in response to infection, inflammation or immunity can also inhibit cancer development and progression. The predominant intracellular signaling pathway triggered by cytokines is the JAK-signal transducer and activator of transcription (STAT) pathway. Knockout mice and clinical human studies have provided evidence that JAK-STAT proteins regulate the immune system, and maintain immune tolerance and tumor surveillance. Moreover, aberrant activation of the JAK-STAT pathways plays an undeniable pathogenic role in several types of human cancers. Thus, in combination, these observations indicate that the JAK-STAT proteins are promising targets for cancer therapy in humans. The data supporting this view are reviewed herein.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
IL-17-Producing Cells in Tumor Immunity: Friends or Foes?
Da-Sol Kuen, Byung-Seok Kim, Yeonseok Chung
Immune Netw. 2020;20(1):e6. doi: 10.4110/in.2020.20.e6.IL-17-Producing Cells in Tumor Immunity: Friends or Foes?
Da-Sol Kuen, Byung-Seok Kim, Yeonseok Chung
Immune Netw. 2020;20(1):. doi: 10.4110/in.2020.20.e6.
Reference
-
1. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444.
Article2. O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013; 368:161–170.3. Spangler JB, Moraga I, Mendoza JL, Garcia KC. Insights into cytokine-receptor interactions from cytokine engineering. Annu Rev Immunol. 2015; 33:139–167.
Article4. Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002; 285:1–24.
Article5. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000; 19:5662–5679.
Article6. Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013; 32:2601–2613.
Article7. Woellner C, Schäffer AA, Puck JM, Renner ED, Knebel C, Holland SM, Plebani A, Grimbacher B. The hyper IgE syndrome and mutations in TYK2. Immunity. 2007; 26:535–536.
Article8. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–1790.
Article9. Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012; 36:515–528.
Article10. O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015; 66:311–328.11. Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010; 32:605–615.
Article12. Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998; 16:293–322.
Article13. Kim JS, Kim YG, Park EJ, Kim B, Lee HK, Hong JT, Kim Y, Han SB. Cell-based immunotherapy for colorectal cancer with cytokine-induced killer cells. Immune Netw. 2016; 16:99–108.
Article14. Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, Griffiths E, Krawczyk C, Richardson CD, Aitken K, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001; 409:349–354.
Article15. David M, Chen HE, Goelz S, Larner AC, Neel BG. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995; 15:7050–7058.
Article16. Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008; 33:122–131.
Article17. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014; 57:5023–5038.
Article18. Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004; 4:97–105.
Article19. Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012; 19:754–759.
Article20. Geron I, Abrahamsson AE, Barroga CF, Kavalerchik E, Gotlib J, Hood JD, Durocher J, Mak CC, Noronha G, Soll RM, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008; 13:321–330.
Article21. Tefferi A. JAK and MPL mutations in myeloid malignancies. Leuk Lymphoma. 2008; 49:388–397.22. Staerk J, Constantinescu SN. The JAK-STAT pathway and hematopoietic stem cells from the JAK2 V617F perspective. JAK-STAT. 2012; 1:184–190.
Article23. Stein BL, Williams DM, Rogers O, Isaacs MA, Spivak JL, Moliterno AR. Disease burden at the progenitor level is a feature of primary myelofibrosis: a multivariable analysis of 164 JAK2 V617F-positive myeloproliferative neoplasm patients. Exp Hematol. 2011; 39:95–101.
Article24. Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, Gilliland DG, Tefferi A. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005; 106:1207–1209.
Article25. Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, Skoda RC. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006; 108:1377–1380.
Article26. Pietra D, Li S, Brisci A, Passamonti F, Rumi E, Theocharides A, Ferrari M, Gisslinger H, Kralovics R, Cremonesi L, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood. 2008; 111:1686–1689.
Article27. Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V, Boiret-Dupré N, Skoda RC, Hermouet S. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006; 108:1865–1867.
Article28. Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, Bass A, Marubayashi S, Heguy A, Garcia-Manero G, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009; 41:455–459.
Article29. Villeval JL, James C, Pisani DF, Casadevall N, Vainchenker W. New insights into the pathogenesis of JAK2 V617F-positive myeloproliferative disorders and consequences for the management of patients. Semin Thromb Hemost. 2006; 32:341–351.
Article30. Jeong EG, Kim SH, Kim MS, Lee SH, Yoo NJ. Absence of JAK2 exon 12 mutation in acute leukemias. Acta Haematol. 2008; 119:38–39.
Article31. Jeong EG, Kim MS, Nam HK, Min CK, Lee S, Chung YJ, Yoo NJ, Lee SH. Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers. Clin Cancer Res. 2008; 14:3716–3721.
Article32. dos Santos NR, Rickman DS, de Reynies A, Cormier F, Williame M, Blanchard C, Stern MH, Ghysdael J. Pre-TCR expression cooperates with TEL-JAK2 to transform immature thymocytes and induce T-cell leukemia. Blood. 2007; 109:3972–3981.
Article33. Kennedy JA, Barabé F, Patterson BJ, Bayani J, Squire JA, Barber DL, Dick JE. Expression of TEL-JAK2 in primary human hematopoietic cells drives erythropoietin-independent erythropoiesis and induces myelofibrosis in vivo . Proc Natl Acad Sci USA. 2006; 103:16930–16935.
Article34. Roncero AM, López-Nieva P, Cobos-Fernández MA, Villa-Morales M, González-Sánchez L, López-Lorenzo JL, Llamas P, Ayuso C, Rodríguez-Pinilla SM, Arriba MC, et al. Contribution of JAK2 mutations to T-cell lymphoblastic lymphoma development. Leukemia. 2016; 30:94–103.
Article35. Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, Lee CK, Gerthner R, Kitamura T, Frantsve J, et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell. 2000; 6:693–704.
Article36. Monni R, Santos SC, Mauchauffe M, Berger R, Ghysdael J, Gouilleux F, Gisselbrecht S, Bernard O, Penard-Lacronique V. The TEL-Jak2 oncoprotein induces Socs1 expression and altered cytokine response in Ba/F3 cells. Oncogene. 2001; 20:849–858.
Article37. dos Santos NR, Ghysdael J. A transgenic mouse model for TEL-JAK2-induced B-cell lymphoma/leukemia. Leukemia. 2006; 20:182–185.
Article38. Onnebo SM, Rasighaemi P, Kumar J, Liongue C, Ward AC. Alternative TEL-JAK2 fusions associated with T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia dissected in zebrafish. Haematologica. 2012; 97:1895–1903.
Article39. Cramer K, Nieborowska-Skorska M, Koptyra M, Slupianek A, Penserga ET, Eaves CJ, Aulitzky W, Skorski T. BCR/ABL and other kinases from chronic myeloproliferative disorders stimulate single-strand annealing, an unfaithful DNA double-strand break repair. Cancer Res. 2008; 68:6884–6888.
Article40. Dierov J, Xu Q, Dierova R, Carroll M. TEL/platelet-derived growth factor receptor beta activates phosphatidylinositol 3 (PI3) kinase and requires PI3 kinase to regulate the cell cycle. Blood. 2002; 99:1758–1765.
Article41. Yokota A, Hirai H, Shoji T, Maekawa T, Okuda K. Constitutively active ABL family kinases, TEL/ABL and TEL/ARG, harbor distinct leukemogenic activities in vivo. Leukemia. 2017.42. Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, Dalton WS. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009; 69:1009–1015.
Article43. Chatterjee M, Stühmer T, Herrmann P, Bommert K, Dörken B, Bargou RC. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood. 2004; 104:3712–3721.
Article44. Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009; 69:6823–6830.
Article45. Liu F, Zhang H, Song H. Upregulation of MEK5 by Stat3 promotes breast cancer cell invasion and metastasis. Oncol Rep. 2017; 37:83–90.
Article46. Dhir R, Ni Z, Lou W, DeMiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate. 2002; 51:241–246.
Article47. Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo . Proc Natl Acad Sci USA. 2000; 97:4227–4232.
Article48. Hang D, Yin Y, Wang L, Yuan H, Du J, Zhu M, Dai J, Chen N, Hu Z, Shen H, et al. Effects of potentially functional polymorphisms in suppressor of cytokine signaling 3 (SOCS3) on the risk of head and neck squamous cancer. J Oral Pathol Med. 2016.49. Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce pd-l1 expression in head and neck cancer. Cancer Res. 2016; 76:1031–1043.
Article50. Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002; 62:3351–3355.51. Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008; 22:686–707.
Article52. Limnander A, Rothman PB. Abl oncogene bypasses normal regulation of Jak/STAT activation. Cell Cycle. 2004; 3:1486–1488.
Article53. Zhang M, Mathews Griner LA, Ju W, Duveau DY, Guha R, Petrus MN, Wen B, Maeda M, Shinn P, Ferrer M, et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc Natl Acad Sci USA. 2015; 112:12480–12485.
Article54. Wen R, Wang D, McKay C, Bunting KD, Marine JC, Vanin EF, Zambetti GP, Korsmeyer SJ, Ihle JN, Cleveland JL. Jak3 selectively regulates Bax and Bcl-2 expression to promote T-cell development. Mol Cell Biol. 2001; 21:678–689.
Article55. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009; 16:91–102.
Article56. Telliez JB, Dowty ME, Wang L, Jussif J, Lin T, Li L, Moy E, Balbo P, Li W, Zhao Y, et al. Discovery of a JAK3-selective inhibitor: functional differentiation of jak3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem Biol. 2016; 11:3442–3451.
Article57. Narla RK, Liu XP, Myers DE, Uckun FM. 4-(3′-Bromo-4'hydroxylphenyl)-amino-6,7-dimethoxyquinazoline: a novel quinazoline derivative with potent cytotoxic activity against human glioblastoma cells. Clin Cancer Res. 1998; 4:1405–1414.58. Zhang H, Zhang YK, Wang YJ, Kathawala RJ, Patel A, Zhu H, Sodani K, Talele TT, Ambudkar SV, Chen ZS, et al. WHI-P154 enhances the chemotherapeutic effect of anticancer agents in ABCG2-overexpressing cells. Cancer Sci. 2014; 105:1071–1078.
Article59. Linwong W, Hirasawa N, Aoyama S, Hamada H, Saito T, Ohuchi K. Inhibition of the antigen-induced activation of rodent mast cells by putative Janus kinase 3 inhibitors WHI-P131 and WHI-P154 in a Janus kinase 3-independent manner. Br J Pharmacol. 2005; 145:818–828.
Article60. Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, Whipple DA, Doty JL, Sun J, Kent CR, et al. The specificity of JAK3 kinase inhibitors. Blood. 2008; 111:2155–2157.
Article61. Stepkowski SM, Erwin-Cohen RA, Behbod F, Wang ME, Qu X, Tejpal N, Nagy ZS, Kahan BD, Kirken RA. Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood. 2002; 99:680–689.
Article62. Rashid S, Bibi N, Parveen Z, Shafique S. Inhibition of Janus kinases by tyrosine phosphorylation inhibitor, Tyrphostin AG-490. J Biomol Struct Dyn. 2015; 33:2368–2379.
Article63. Sewgobind VD, Quaedackers ME, van der Laan LJ, Kraaijeveld R, Korevaar SS, Chan G, Weimar W, Baan CC. The Jak inhibitor CP-690,550 preserves the function of CD4CD25FoxP3 regulatory T cells and inhibits effector T cells. Am J Transplant. 2010; 10:1785–1795.
Article64. Ju W, Zhang M, Jiang JK, Thomas CJ, Oh U, Bryant BR, Chen J, Sato N, Tagaya Y, Morris JC, et al. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood. 2011; 117:1938–1946.
Article65. Hart S, Goh KC, Novotny-Diermayr V, Tan YC, Madan B, Amalini C, Ong LC, Kheng B, Cheong A, Zhou J, et al. Pacritinib (SB1518), a JAK2/FLT3 inhibitor for the treatment of acute myeloid leukemia. Blood Cancer J. 2011; 1:e44.
Article66. Verstovsek S, Tam CS, Wadleigh M, Sokol L, Smith CC, Bui LA, Song C, Clary DO, Olszynski P, Cortes J, et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor. Leuk Res. 2014; 38:316–322.
Article67. McCarthy AA. Exelixis: integrated drug-discovery and development platform for human therapeutics. Chem Biol. 2005; 12:407–408.
Article68. Moran N. Incyte comes of age with JAK inhibitor approval. Nat Biotechnol. 2012; 30:3–5.
Article69. Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011; 29:789–796.
Article70. Hexner EO, Serdikoff C, Jan M, Swider CR, Robinson C, Yang S, Angeles T, Emerson SG, Carroll M, Ruggeri B, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008; 111:5663–5671.
Article71. Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010; 363:1117–1127.
Article72. Shi JG, Chen X, Emm T, Scherle PA, McGee RF, Lo Y, Landman RR, McKeever EG Jr, Punwani NG, Williams WV, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol. 2012; 52:809–818.
Article73. Aubert L, Guilbert M, Corbet C, Génot E, Adriaenssens E, Chassat T, Bertucci F, Daubon T, Magné N, Le Bourhis X, et al. NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib. Oncotarget. 2015; 6:9807–9819.
Article74. Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, Clark R, Levis MJ, Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006; 108:3262–3270.
Article75. Hexner EO, Mascarenhas J, Prchal J, Roboz GJ, Baer MR, Ritchie EK, Leibowitz D, Demakos EP, Miller C, Siuty J, et al. Phase I dose escalation study of lestaurtinib in patients with myelofibrosis. Leuk Lymphoma. 2015; 56:2543–2551.
Article76. Minturn JE, Evans AE, Villablanca JG, Yanik GA, Park JR, Shusterman S, Groshen S, Hellriegel ET, Bensen-Kennedy D, Matthay KK, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol. 2011; 68:1057–1065.
Article77. Diaz T, Navarro A, Ferrer G, Gel B, Gaya A, Artells R, Bellosillo B, Garcia-Garcia M, Serrano S, Martínez A, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One. 2011; 6:e18856.
Article78. Soderquist C, Bagg A. Coexistent BCR-ABL1 and JAK2 V617F: converting CML dwarves to ET staghorns with imatinib therapy. Blood. 2014; 124:2463.
Article79. Qian XL, Zhang J, Li PZ, Lang RG, Li WD, Sun H, Liu FF, Guo XJ, Gu F, Fu L. Dasatinib inhibits c-src phosphorylation and prevents the proliferation of Triple-Negative Breast Cancer (TNBC) cells which overexpress Syndecan-Binding Protein (SDCBP). PLoS One. 2017; 12:e0171169.
Article80. Zhou A, Knoche EM, Engle EK, Fisher DA, Oh ST. Concomitant JAK2 V617F-positive polycythemia vera and BCR-ABL-positive chronic myelogenous leukemia treated with ruxolitinib and dasatinib. Blood Cancer J. 2015; 5:e351.
Article81. Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008; 134:379.
Article82. Rahmani M, Aust MM, Attkisson E, Williams DC Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012; 119:6089–6098.
Article83. Huynh H, Ngo VC, Choo SP, Poon D, Koong HN, Thng CH, Toh HC, Zheng L, Ong LC, Jin Y, et al. Sunitinib (SUTENT, SU11248) suppresses tumor growth and induces apoptosis in xenograft models of human hepatocellular carcinoma. Curr Cancer Drug Targets. 2009; 9:738–747.
Article84. Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res. 2010; 8:35–45.
Article85. Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011; 11:856–861.
Article86. Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010; 33:991–998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in the Development of TGF-β Signaling Inhibitors for Anticancer Therapy
- The role of soluble common gamma chain in autoimmune disease
- Negative Regulation of Intracellular Cytokine Signal Transduction
- C-Reactive Protein Signaling Pathways in Tumor Progression
- The Role of STAT1 in T Helper Cell Differentiation during Breast Cancer Progression