Nomogram Predicting Clinical Outcomes in Non-small Cell Lung Cancer Patients Treated with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. kimdw@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2380369
- DOI: http://doi.org/10.4143/crt.2013.120
Abstract
- PURPOSE
The aim of this study was to develop a pragmatic nomogram for prediction of progressionfree survival (PFS) for the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) in EGFR mutant non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
A total of 306 recurred or metastatic NSCLC patients with EGFR mutation, who received EGFR TKIs, were enrolled in this study. We developed the nomogram, using a Cox proportional hazard regression model for PFS.
RESULTS
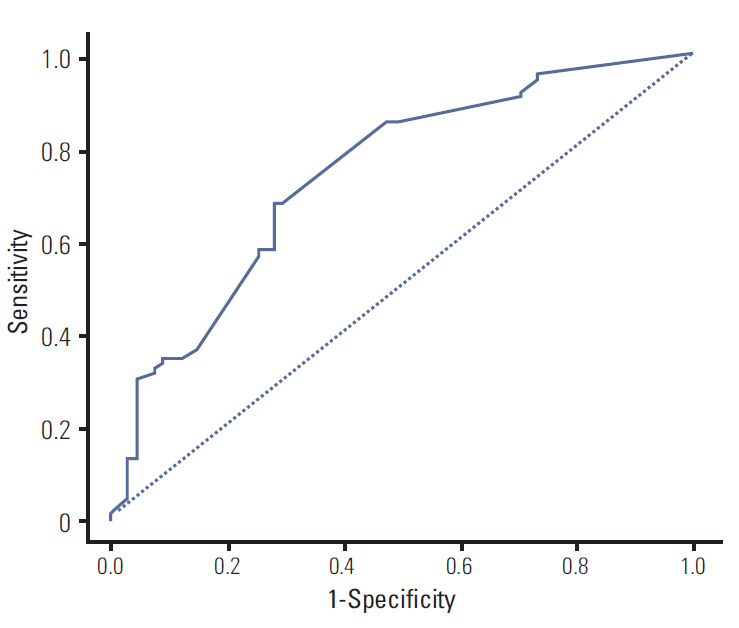
The median PFS was 11.2 months. Response rate to EGFR TKI was 71.9%. Multivariate Cox model identified disease status, performance status, chemotherapy line, response to EGFR TKI, and bone metastasis as independent prognostic factors, and the nomogram for PFS was developed, based on these covariates. The concordance index for a nomogram was 0.708, and the calibration was also good.
CONCLUSION
We developed a nomogram, based on clinical characteristics, for prediction of the PFS to EGFR TKI in NSCLC patients with EGFR mutation.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Prognostic Scoring Index for Patients with Metastatic Pancreatic Adenocarcinoma
Hyung Soon Park, Hye Sun Lee, Ji Soo Park, Joon Seong Park, Dong Ki Lee, Se-Joon Lee, Dong Sup Yoon, Min Goo Lee, Hei-Cheul Jeung
Cancer Res Treat. 2016;48(4):1253-1263. doi: 10.4143/crt.2015.400.Prognostic Factors and Scoring Model for Survival in Metastatic Biliary Tract Cancer
Hyung Soon Park, Ji Soo Park, You Jin Chun, Yun Ho Roh, Jieun Moon, Hong Jae Chon, Hye Jin Choi, Joon Seong Park, Dong Ki Lee, Se-Joon Lee, Dong Sup Yoon, Hei-Cheul Jeung
Cancer Res Treat. 2017;49(4):1127-1139. doi: 10.4143/crt.2016.538.The Risk of Herpes Zoster in Patients with Non-small Cell Lung Cancer according to Chemotherapy Regimens: Tyrosine Kinase Inhibitors versus Cytotoxic Chemotherapy
Ji Young Choi, Miso Kim, Bhumsuk Keam, Tae Min Kim, Dong-Wan Kim, Dae Seog Heo, Seong Jin Jo
Cancer Res Treat. 2019;51(1):169-177. doi: 10.4143/crt.2017.491.
Reference
-
References
1. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.
Article2. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–500.3. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005; 23:2493–501.
Article4. Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005; 23:6829–37.
Article5. Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005; 23:2513–20.
Article6. Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, McShane LM, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011; 29:2121–7.
Article7. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009; 27:6251–66.
Article8. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article9. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article10. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol. 2013 Aug 6 [Epub]. http://dx.doi.org/10.1007/s10147-013-0602-1.
Article11. Kim YT, Kim TY, Lee DS, Park SJ, Park JY, Seo SJ, et al. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008; 59:111–8.
Article12. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.13. Keam B, Im SA, Park S, Nam BH, Han SW, Oh DY, et al. Nomogram predicting clinical outcomes in breast cancer patients treated with neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2011; 137:1301–8.
Article14. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008; 26:1364–70.
Article15. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–87.
Article16. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143:29–36.
Article17. Nam BH. Discrimination and calibration in survival analysis [Dissertation]. Boston: Boston University; 2000.18. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004; 23:2109–23.19. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000; 56:337–44.
Article20. Florescu M, Hasan B, Seymour L, Ding K, Shepherd FA; National Cancer Institute of Canada Clinical Trials Group. A clinical prognostic index for patients treated with erlotinib in National Cancer Institute of Canada Clinical Trials Group study BR.21. J Thorac Oncol. 2008; 3:590–8.
Article21. Kim ST, Lee J, Sun JM, Park YH, Ahn JS, Park K, et al. Prognostic model to predict outcomes in non-small cell lung cancer patients with erlotinib as salvage treatment. Oncology. 2010; 79:78–84.
Article22. Chung KP, Huang YT, Chang YL, Yu CJ, Yang CH, Chang YC, et al. Clinical significance of thyroid transcription factor-1 in advanced lung adenocarcinoma under epidermal growth factor receptor tyrosine kinase inhibitor treatment. Chest. 2012; 141:420–8.
Article23. Nose N, Uramoto H, Iwata T, Hanagiri T, Yasumoto K. Expression of estrogen receptor beta predicts a clinical response and longer progression-free survival after treatment with EGFR-TKI for adenocarcinoma of the lung. Lung Cancer. 2011; 71:350–5.
Article24. Ludovini V, Bianconi F, Pistola L, Chiari R, Minotti V, Colella R, et al. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011; 6:707–15.
Article25. Han SW, Kim TY, Jeon YK, Hwang PG, Im SA, Lee KH, et al. Optimization of patient selection for gefitinib in non-small cell lung cancer by combined analysis of epidermal growth factor receptor mutation, K-ras mutation, and Akt phosphorylation. Clin Cancer Res. 2006; 12:2538–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Overview of ALK and ROS1 Rearranged Lung Cancer
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Current Status of Immunotherapy for Lung Cancer and Future Perspectives
- Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor