Cancer Res Treat.
2013 Jun;45(2):79-85.
Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Division of Pulmonary, Allergy and Critical Care Medicine of Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 3Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea. kyc0923@chonnam.ac.kr
Abstract
- Since the first description of non-small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutation as a distinct clinical entity, studies have proved EGFR tyrosine kinase inhibitors (TKIs) as a first choice of treatment. The median response duration of TKIs as a first-line treatment for EGFR mutant tumors ranges from 11 to 14 months. However, acquired resistance to EGFR-TKIs is inevitable due to various mechanisms, such as T790M, c-Met amplification, activation of alternative pathways (IGF-1, HGF, PI3CA, AXL), transformation to mesenchymal cell or small cell features, and tumor heterogeneity. Until development of a successful treatment strategy to overcome such acquired resistance, few options are currently available. Here we provide a summary of the therapeutic options after failure of first line EGFR-TKI treatment for NSCLC.
MeSH Terms
Figure
Reference
-
1. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139. PMID: 15118073.
Article2. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–2388. PMID: 20573926.
Article3. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957. PMID: 19692680.
Article4. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742. PMID: 21783417.
Article5. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009; 361:958–967. PMID: 19692684.
Article6. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012; 44:852–860. PMID: 22751098.
Article7. Nurwidya F, Takahashi F, Murakami A, Takahashi K. Epithelial mesenchymal transition in drug resistance and metastasis of lung cancer. Cancer Res Treat. 2012; 44:151–156. PMID: 23091440.
Article8. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.
Article9. Saijo N. Present status and problems on molecular targeted therapy of cancer. Cancer Res Treat. 2012; 44:1–10. PMID: 22500155.
Article10. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012; 13:528–538. PMID: 22452896.
Article11. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Lung Cancer Screening. Fort Washington: National Comprehensive Cancer Network;2013.12. Wu SG, Yang CH, Yu CJ, Lee JH, Hsu YC, Chang YL, et al. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer. 2011; 72:333–339. PMID: 21111508.
Article13. Park JH, Lee SH, Keam B, Kim TM, Kim DW, Yang SC, et al. EGFR mutations as a predictive marker of cytotoxic chemotherapy. Lung Cancer. 2012; 77:433–437. PMID: 22521649.
Article14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
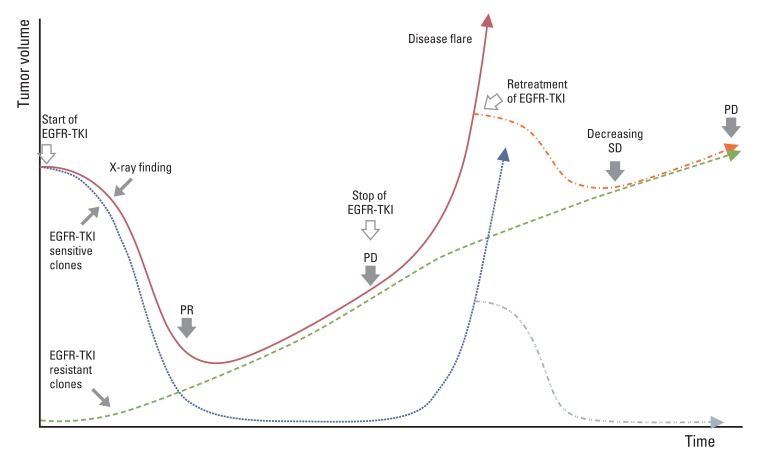
Article15. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011; 17:6298–6303. PMID: 21856766.
Article16. Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007; 13:5150–5155. PMID: 17785570.
Article17. Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. 2013; 79:33–39. PMID: 23079155.
Article18. Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006; 24:4517–4520. PMID: 16983123.
Article19. Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011; 29:e443–e445. PMID: 21422405.
Article20. Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013; 8:346–351. PMID: 23407558.
Article21. Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001; 19:3267–3279. PMID: 11432895.
Article22. Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002; 20:4292–4302. PMID: 12409327.
Article23. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–132. PMID: 16014882.
Article24. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–1537. PMID: 16257339.
Article25. Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007; 25:2528–2533. PMID: 17577030.
Article26. Lee DH, Kim SW, Suh C, Yoon DH, Yi EJ, Lee JS. Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol. 2008; 19:2039–2042. PMID: 18644828.
Article27. Viswanathan A, Pillot G, Govindan R. Lack of response to erlotinib after progression on gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2005; 50:417–418. PMID: 16129510.
Article28. Costa DB, Nguyen KS, Cho BC, Sequist LV, Jackman DM, Riely GJ, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008; 14:7060–7067. PMID: 18981003.
Article29. Sim SH, Han SW, Oh DY, Lee SH, Kim DW, Im SA, et al. Erlotinib after Gefitinib failure in female never-smoker Asian patients with pulmonary adenocarcinoma. Lung Cancer. 2009; 65:204–207. PMID: 19110337.
Article30. Wong AS, Soong R, Seah SB, Lim SW, Chuah KL, Nga ME, et al. Evidence for disease control with erlotinib after gefitinib failure in typical gefitinib-sensitive Asian patients with non-small cell lung cancer. J Thorac Oncol. 2008; 3:400–404. PMID: 18379359.
Article31. Zhou ZT, Xu XH, Wei Q, Lu MQ, Wang J, Wen CH. Erlotinib in advanced non-small-cell lung cancer after gefitinib failure. Cancer Chemother Pharmacol. 2009; 64:1123–1127. PMID: 19322567.
Article32. Vasile E, Tibaldi C, Chella A, Falcone A. Erlotinib after failure of gefitinib in patients with advanced non-small cell lung cancer previously responding to gefitinib. J Thorac Oncol. 2008; 3:912–914. PMID: 18670311.
Article33. Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, et al. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011; 47:2603–2606. PMID: 21784628.
Article34. Yokouchi H, Yamazaki K, Kinoshita I, Konishi J, Asahina H, Sukoh N, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007; 7:51. PMID: 17374153.
Article35. Oh IJ, Ban HJ, Kim KS, Kim YC. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer. 2012; 77:121–127. PMID: 22333554.
Article36. Asahina H, Oizumi S, Inoue A, Kinoshita I, Ishida T, Fujita Y, et al. Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology. 2010; 79:423–429. PMID: 21474967.
Article37. Ramalingam SS, Blackhall F, Krzakowski M, Barrios CH, Park K, Bover I, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012; 30:3337–3344. PMID: 22753918.
Article38. Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SH, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012; 13:539–548. PMID: 22452895.
Article39. Kwak E. The role of irreversible HER family inhibition in the treatment of patients with non-small cell lung cancer. Oncologist. 2011; 16:1498–1507. PMID: 22016476.
Article40. Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009; 462:1070–1074. PMID: 20033049.
Article41. Janjigian YY, Groen HJ, Horn L, Smit EF, Fu Y, Wang F, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib. J Clin Oncol. 2011; 29(S):7525.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overview of ALK and ROS1 Rearranged Lung Cancer
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Mechanisms of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance and Strategies to Overcome Resistance in Lung Adenocarcinoma
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Systemic Nocardiosis Mimicking Disease Flare-up after Discontinuation of Gefitinib in a Patient with EGFR-Mutant Lung Cancer