Ann Surg Treat Res.
2017 May;92(5):348-354. 10.4174/astr.2017.92.5.348.
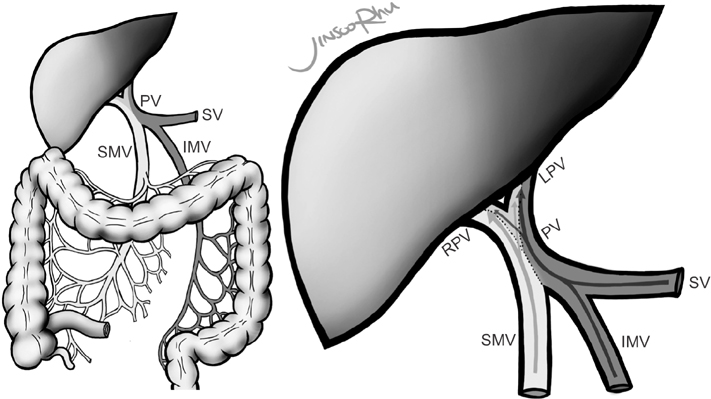
Streamline flow of the portal vein affects the lobar distribution of colorectal liver metastases and has a clinical impact on survival
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. chdkwon@skku.edu
- KMID: 2377705
- DOI: http://doi.org/10.4174/astr.2017.92.5.348
Abstract
- PURPOSE
It is believed that blood from the superior mesenteric vein and splenic vein mixes incompletely in the portal vein and maintains a streamline flow influencing its anatomic distribution. Although several experimental studies have demonstrated the existence of streamlining, clinical studies have shown conflicting results. We investigated whether streamlining of portal vein affects the lobar distribution of colorectal liver metastases and estimated its impact on survival.
METHODS
Data of patients who underwent hepatectomy for colorectal liver metastases were retrospectively collected. The chi-square test was used for analyzing the distribution of metastasis. Cox analysis was used to identify risk factors of survival. Fisher exact test was used for subgroup analysis comparing hepatic recurrence.
RESULTS
A total of 410 patients were included. The right-to-left ratio of liver metastases were 2.20:1 in right-sided colon cancer and 1.39:1 in left-sided cancer (P = 0.017). Cox analyses showed that margin < 5 mm (P < 0.001; 95% confidence interval [CI], 1.648-4.884; hazard ratio [HR], 2.837), age ≥ 60 years (P = 0.004; 95% CI, 1.269-3.641; HR, 2.149), N2 status (P < 0.001, 95% CI, 1.598-4.215; HR, 2.595), tumor size ≥ 45 mm (P = 0.014; 95% CI, 1.159-3.758; HR, 2.087) and other metastasis (P = 0.012; 95% CI, 1.250-5.927; HR, 2.722) were risk factors of survival. However, in 70 patients who underwent right hemihepatectomy for solitary metastasis, left-sided colorectal cancer was a risk factor (P = 0.019; 95% CI, 1.293-17.956; HR, 4.818), and was associated with higher recurrence than right-sided cancer (43.1% and 15.8%, respectively, P = 0.049).
CONCLUSION
This study showed significant difference in lobar distribution of liver metastases between right colon cancer and left colorecral cancer. Furthermore, survival of left-sided colorectal cancer was poorer than that of right-sided cancer in patients who underwent right hemihepatectomy for solitary metastasis. These findings can be helpful for clinicians planning treatment strategy.
MeSH Terms
Figure
Cited by 2 articles
-
Laparoscopy of hepatocellular carcinoma is helpful in minimizing intra-abdominal adhesion during salvage transplantation
Jinsoo Rhu, Jong Man Kim, Gyu Seong Choi, Choon Hyuck David Kwon, Jae-Won Joh, Olivier Soubrane
Ann Surg Treat Res. 2018;95(5):258-266. doi: 10.4174/astr.2018.95.5.258.Metastasis-Directed Local Therapy of Hepatic Oligometastasis from Colorectal Cancer and Future Perspective in Radiation Therapy
Gyu Sang Yoo, Chai Hong Rim, Won Kyung Cho, Jae-Uk Jeong, Eui Kyu Chie, Hyeon-Min Cho, Jun Won Um, Yong Chan Ahn, Jong Hoon Lee
Cancer Res Treat. 2023;55(3):707-719. doi: 10.4143/crt.2022.1599.
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Tocchi A, Mazzoni G, Brozzetti S, Miccini M, Cassini D, Bettelli E. Hepatic resection in stage IV colorectal cancer: prognostic predictors of outcome. Int J Colorectal Dis. 2004; 19:580–585.3. Morise Z, Sugioka A, Fujita J, Hoshimoto S, Kato T, Hasumi A, et al. Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastrointest Surg. 2006; 10:6–11.4. Lee H, Heo JS, Cho YB, Yun SH, Kim HC, Lee WY, et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: a propensity score analysis. World J Gastroenterol. 2015; 21:3300–3307.5. Copher GH, Dick BM. “Streamline” phenomena in the portal vein and selective distribution of portal blood in the liver. Arch Surg. 1928; 17:408–419.6. Desai AG, Park CH, Schilling JF. “Streaming” in portal vein. Its effect on the spread of metastases to the liver. Clin Nucl Med. 1985; 10:556–559.7. Moore GE, Bridenbaugh RB. Roentgen demonstration of the venous circulation in the liver; portal venography. Radiology. 1951; 57:685–690.8. Incedayı M, Arıbal S, Sivrioglu AK, Sonmez G, Ozturk E, Yalcın B, et al. Are hepatic portal venous system components distributed equally in the liver? A multidetector computerized tomography study. Balkan Med J. 2012; 29:419–423.9. Dionne L. The pattern of blood-borne metastasis from carcinoma of rectum. Cancer. 1965; 18:775–781.10. Konopke R, Distler M, Ludwig S, Kersting S. Location of liver metastases reflects the site of the primary colorectal carcinoma. Scand J Gastroenterol. 2008; 43:192–195.11. Schulz W, Hagen C, Hort W. The distribution of liver metastases from colonic cancer. A quantitative postmortem study. Virchows Arch A Pathol Anat Histopathol. 1985; 406:279–284.12. Shirai Y, Wakai T, Ohtani T, Sakai Y, Tsukada K, Hatakeyama K. Colorectal carcinoma metastases to the liver. Does primary tumor location affect its lobar distribution? Cancer. 1996; 77:2213–2216.13. Wigmore SJ, Madhavan K, Redhead DN, Currie EJ, Garden OJ. Distribution of colorectal liver metastases in patients referred for hepatic resection. Cancer. 2000; 89:285–287.14. Strohmeyer T, Schultz W. The distribution of metastases of different primary tumors in the liver. Liver. 1986; 6:184–187.15. Um EH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Calculation of standard liver volume in Korean adults with analysis of confounding variables. Korean J Hepatobiliary Pancreat Surg. 2015; 19:133–138.16. Cady B, Stone MD, McDermott WV Jr, Jenkins RL, Bothe A Jr, Lavin PT, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992; 127:561–568.17. Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986; 73:727–731.18. Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994; 116:703–710.19. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Association Francaise de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996; 77:1254–1262.20. Fortner JG, Silva JS, Cox EB, Golbey RB, Gallowitz H, Maclean BJ. Multivariate analysis of a personal series of 247 patients with liver metastases from colorectal cancer. II. Treatment by intrahepatic chemotherapy. Ann Surg. 1984; 199:317–324.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver Transplantation in Liver Cirrhosis Patients Accompanied by Portal Vein Thrombosis
- Blood flow volume difference (P-SS) between the portal vein and thesum of splenic vein and superior mesenteric vein in portal hypertension
- More than 7-year survival of a patient following repeat hepatectomy for total 20 colon cancer liver metastases
- Portal vein fenestration: a case report of an unusual portal vein developmental anomaly
- Preduodenal Portal Vein Associated with Duodenal Obstruction: A case report