Korean Circ J.
2017 Mar;47(2):182-192. 10.4070/kcj.2015.0295.
AT1 Receptor Modulator Attenuates the Hypercholesterolemia-Induced Impairment of the Myocardial Ischemic Post-Conditioning Benefits
- Affiliations
-
- 1Department of Cardiology, Henan University Huaihe Hospital, Henan, China. drliyanming86@hotmail.com
- KMID: 2377458
- DOI: http://doi.org/10.4070/kcj.2015.0295
Abstract
- BACKGROUND AND OBJECTIVES
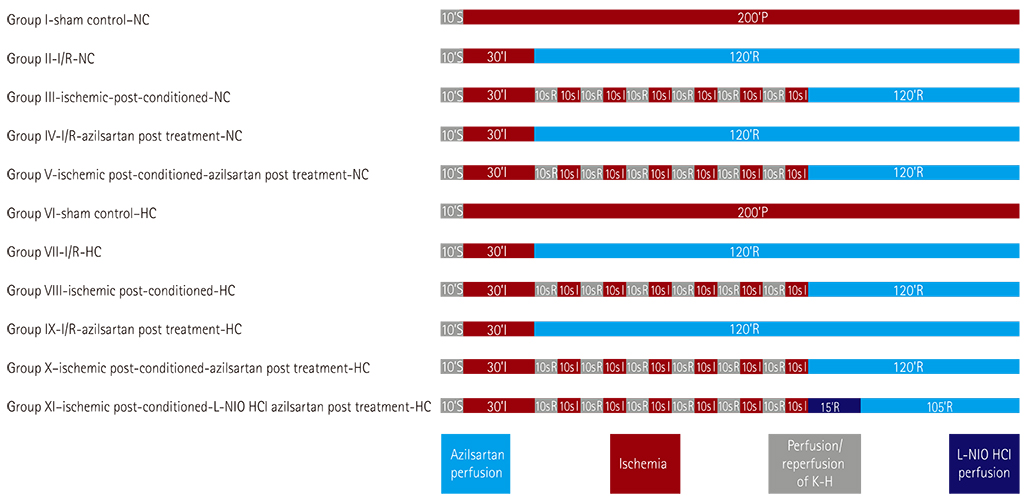
Ischemic post-conditioning (PostC) has been demonstrated as a novel strategy to harness nature's protection against myocardial ischemia-reperfusion (I/R). Hypercholesterolemia (HC) has been reported to block the effect of PostC on the heart. Angiotensin II type-1 (AT1) modulators have shown benefits in myocardial ischemia. The present study investigates the effect of a novel inhibitor of AT1, azilsartan in PostC of the heart of normocholesterolemic (NC) and HC rats.
MATERIALS AND METHODS
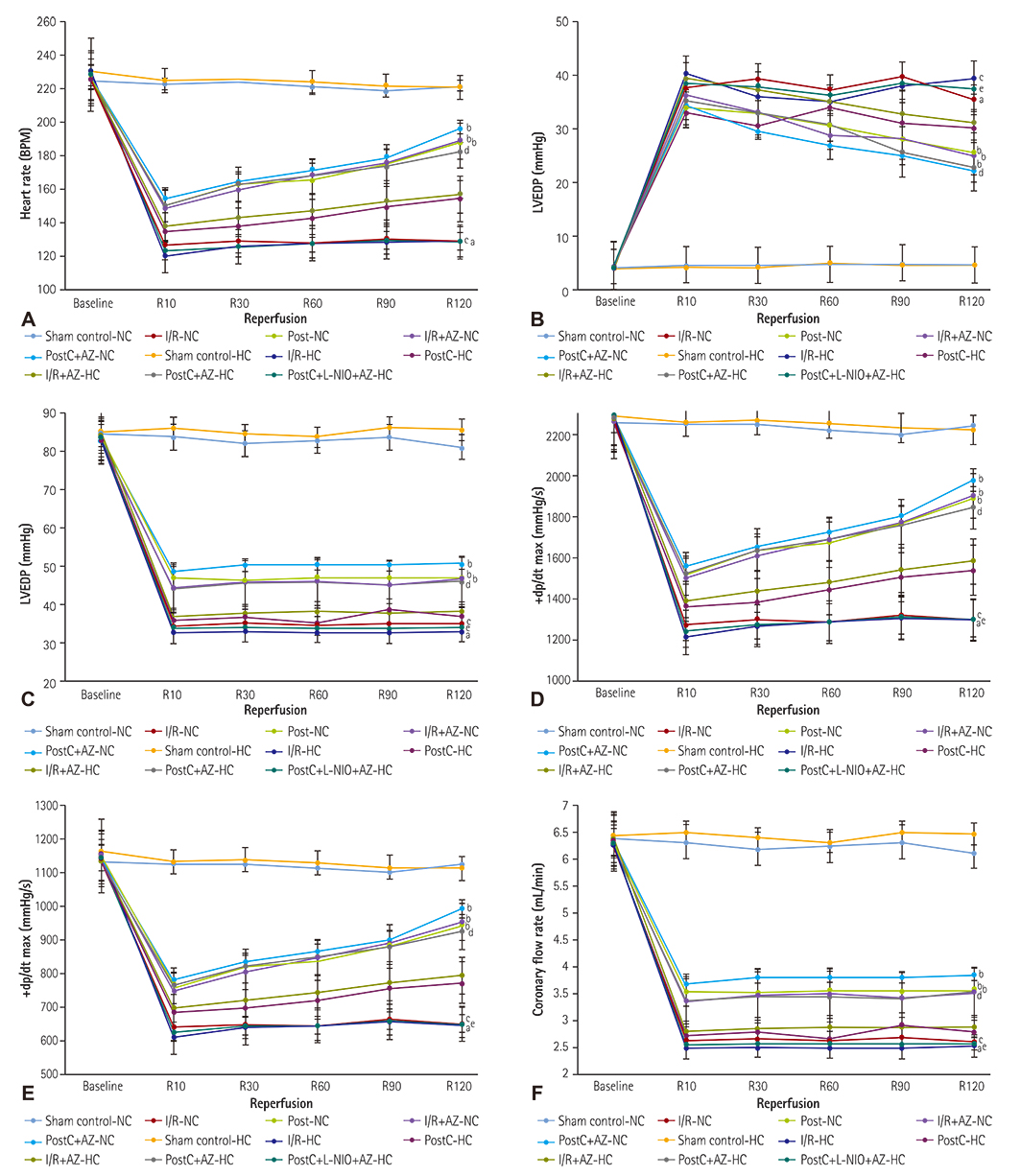
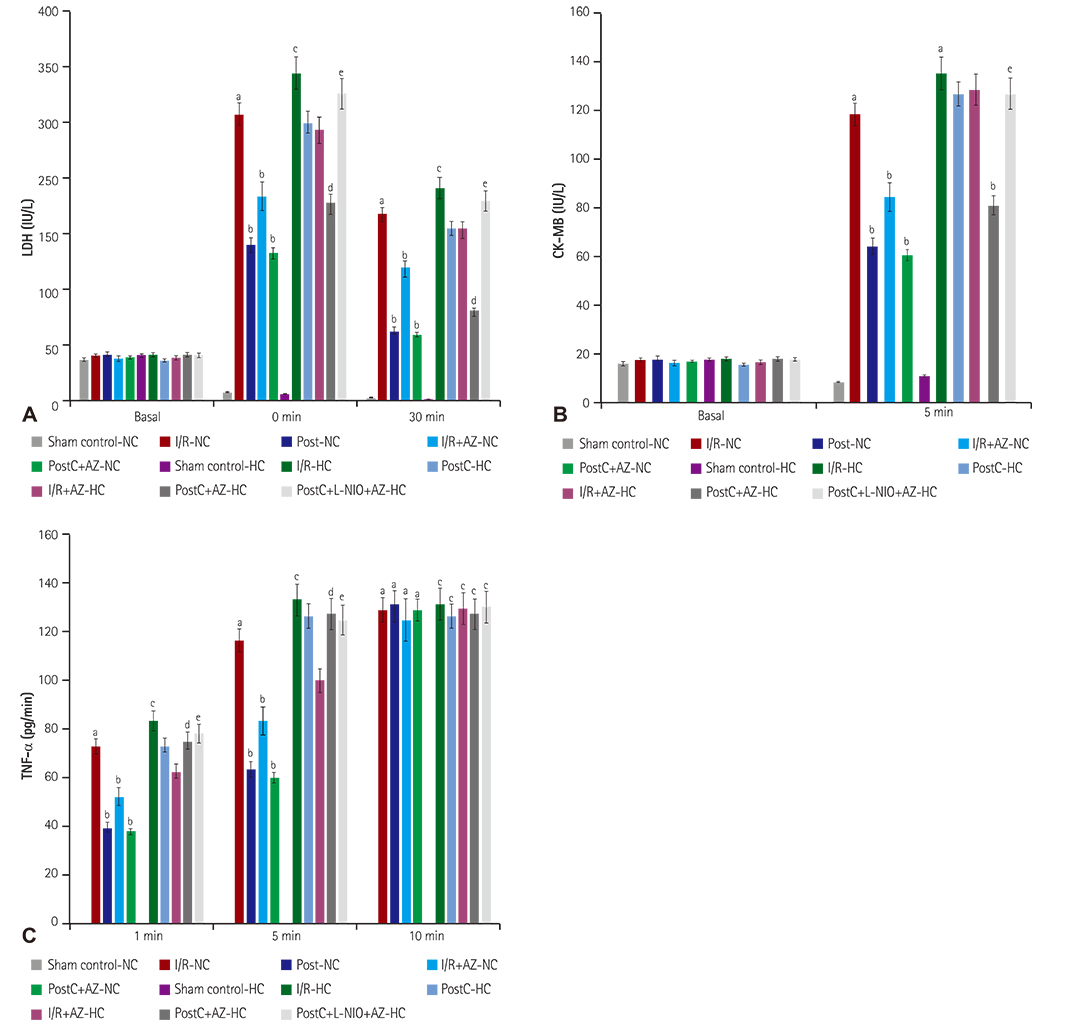
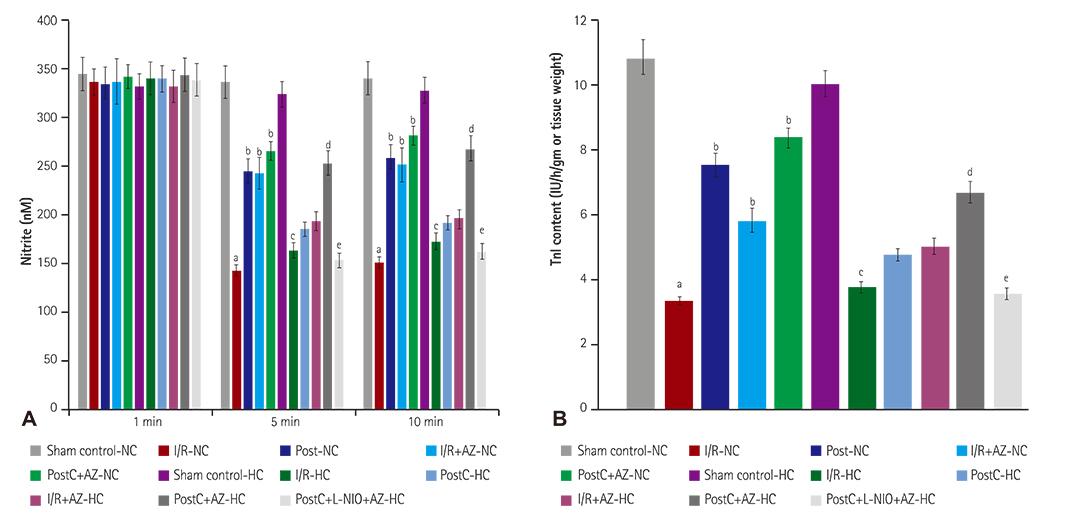
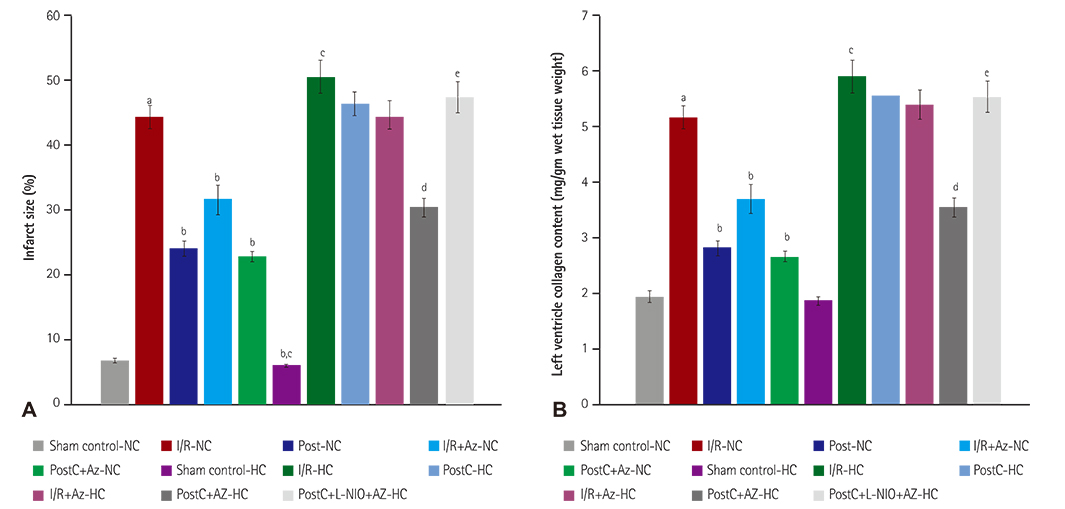
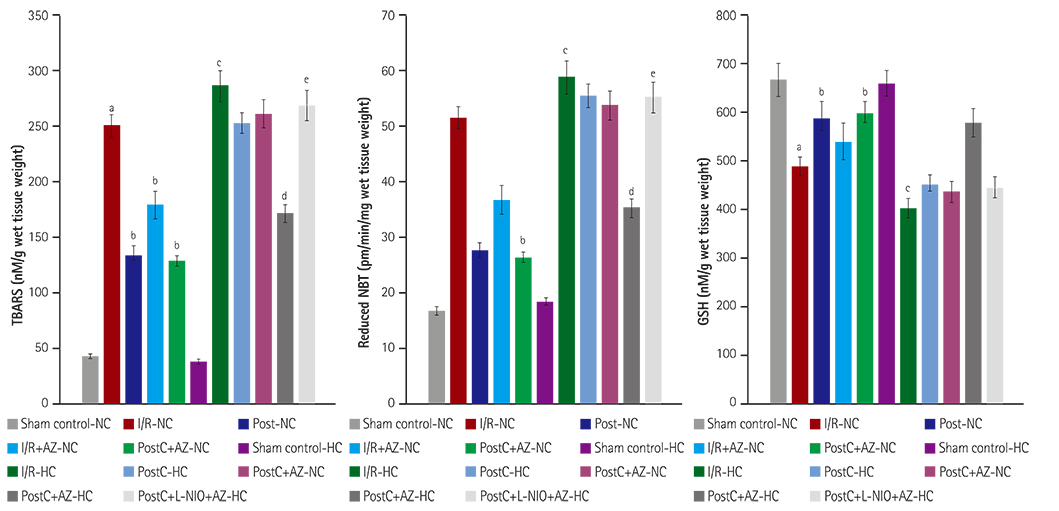
HC was induced by the administration of high-fat diet to the animals for eight weeks. Isolated Langendorff's perfused NC and HC rat hearts were exposed to global ischemia for 30 min and reperfusion for 120 min. I/R-injury had been assessed by cardiac hemodynamic parameters, myocardial infarct size, release of tumor necrosis factor-alpha troponin I, lactate dehydrogenase, creatine kinase, nitrite in coronary effluent, thiobarbituric acid reactive species, a reduced form of glutathione, superoxide anion, and left ventricle collagen content in normal and HC rat hearts.
RESULTS
Azilsartan post-treatment and six episodes of PostC (10 sec each) afforded cardioprotection against I/R-injury in normal rat hearts. PostC protection against I/R-injury was abolished in HC rat hearts. Azilsartan prevented the HC-mediated impairment of the beneficial effects of PostC in I/R-induced myocardial injury, which was inhibited by L-Nâµ-(1-Iminoethyl)ornithinehydrochloride, a potent inhibitor of endothelial nitric oxide synthase (eNOS).
CONCLUSION
Azilsartan treatment has attenuated the HC-induced impairment of beneficial effects of PostC in I/R-injury of rat hearts, by specifically modulating eNOS. Azilsartan may be explored further in I/R-myocardial injury, both in NC and HC conditions, with or without PostC.
MeSH Terms
-
Angiotensin II
Animals
Collagen
Creatine Kinase
Diet, High-Fat
Glutathione
Heart
Heart Ventricles
Hemodynamics
Hypercholesterolemia
Ischemia
Ischemic Postconditioning*
L-Lactate Dehydrogenase
Myocardial Infarction
Myocardial Ischemia
Nitric Oxide Synthase Type III
Rats
Reperfusion
Reperfusion Injury
Superoxides
Troponin I
Tumor Necrosis Factor-alpha
Angiotensin II
Collagen
Creatine Kinase
Glutathione
L-Lactate Dehydrogenase
Nitric Oxide Synthase Type III
Superoxides
Troponin I
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Jivraj N, Liew F, Marber M. Ischaemic postconditioning: cardiac protection after the event. Anaesthesia. 2015; 70:598–612.2. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004; 61:448–460.3. Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009; 47:32–40.4. Wu N, Zhang X, Jia P, Jia D. Hypercholesterolemia abrogates the protective effect of ischemic postconditioning by induction of apoptosis and impairment of activation of reperfusion injury salvage kinase pathway. Biochem Biophys Res Commun. 2015; 458:148–153.5. Wu N, Li W, Shu W, Lv Y, Jia D. Inhibition of Rho-kinase by fasudil restores the cardioprotection of ischemic postconditioninng in hypercholesterolemic rat heart. Mol Med Rep. 2014; 10:2517–2524.6. Ferrario CM, Smith R, Levy P, Strawn W. The hypertension-lipid connection: insights into the relation between angiotensin II and cholesterol in atherogenesis. Am J Med Sci. 2002; 323:17–24.7. Li KY, Zhang YJ. Valsartan-induced cardioprotection involves angiotensin II type 2 receptorupregulation in isolated ischaemia and reperfused rat hearts. Acta Cardiol. 2015; 70:67–72.8. Skorska A, von Haehling S, Ludwig M, et al. The CD4(+) AT2R(+) T cell subpopulation improves post-infarction remodelling and restores cardiac function. J Cell Mol Med. 2015; 19:1975–1985.9. Kansal SK, Jyoti U, Sharma S, Kaura A, Deshmukh R, Goyal S. Effect of zinc supplements in the attenuated cardioprotective effect of ischemic preconditioning in hyperlipidemic rat heart. Naunyn Schmiedebergs Arch Pharmacol. 2015; 388:635–641.10. Badalzadeh R, Yousefi B, Majidinia M, Ebrahimi H. Anti-arrhythmic effect of diosgenin in reperfusion-induced myocardial injury in a rat model: activation of nitric oxide system and mitochondrial KATP channel. J Physiol Sci. 2014; 64:393–400.11. Miura S, Matsuo Y, Nakayama A, Tomita S, Suematsu Y, Saku K. Ability of the new AT1 receptor blocker azilsartan to block angiotensin II-induced AT1 receptor activation after wash-out. J Renin Angiotensin Aldosterone Syst. 2014; 15:7–12.12. Matsumoto S, Shimabukuro M, Fukuda D, et al. Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser(1177)/Thr(497) of endothelial nitric oxide synthase in diabetic mice. Cardiovasc Diabetol. 2014; 13:30.13. Parikh V, Singh M. Possible role of cardiac mast cell degranulation and preservation of nitric oxide release in isolated rat heart subjected to ischemic preconditioning. Mol Cell Biochem. 1999; 199:1–6.14. Sato T, Sato H, Oguchi T, et al. Insulin preconditioning elevates p-Akt and cardiac contractility after reperfusion in the isolated ischemic rat heart. Biomed Res Int. 2014; 2014:536510.15. Jamal IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981; 112:70–75.16. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.17. Wang HD, Pagano PJ, Du Y, et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998; 82:810–818.18. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963; 61:882–888.19. Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006; 69:178–185.20. Xu T, Tang H, Zhang B, et al. Exercise preconditioning attenuates pressure overload-induced pathological cardiac hypertrophy. Int J Clin Exp Pathol. 2015; 8:530–540.21. Carroll MA, Kang Y, Chander PN, Stier CT Jr. Azilsartan is associated with increased circulating angiotensin-(1-7) levels and reduced renovascular 20-HETE levels. Am J Hypertens. 2015; 28:664–671.22. Hye Khan MA, Neckář J, Cummens B, Wahl GM, Imig JD. Azilsartan decreases renal and cardiovascular injury in the spontaneously hypertensive obese rat. Cardiovasc Drugs Ther. 2014; 28:313–322.23. Iwanami J, Mogi M, Tsukuda K, et al. Direct angiotensin II type 2 receptor stimulation by compound 21 prevents vascular dementia. J Am Soc Hypertens. 2015; 9:250–256.24. Tarikuz Zaman AK, McLean DL, Sobel BE. The efficacy and tolerability of azilsartan in obese insulin-resistant mice with left ventricular pressure overload. J Cardiovasc Pharmacol. 2013; 62:381–387.25. Summary Basis of Decision-SBD for PrEDARBI [Internet]. Canada: Azilsartan medoxomil, Bureau of Cardiology, Allergy and Neurological Sciences, Health Canada;2012. 06. cited 2016 Jan. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbdsmd/drug-med/sbd_smd_2012_edarbi_145305-eng.php.26. Sgarra L, Leo V, Addabbo F, et al. Intermittent losartan administration triggers cardiac post-conditioning in isolated rat hearts: role of BK2 receptors. PLoS One. 2014; 9:e88542.27. Iwai M, Chen R, Imura Y, Horiuchi M. TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens. 2007; 20:579–586.28. Nakamura Y, Suzuki S, Saitoh S, Takeishi Y. New angiotensin II type 1 receptor blocker, azilsartan, attenuates cardiac remodeling after myocardial infarction. Biol Pharm Bull. 2013; 36:1326–1331.29. Yang JT, Qian LB, Zhang FJ, et al. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J Cardiovasc Pharmacol. 2015; 65:349–356.30. Huisamen B, Pêrel SJ, Friedrich SO, Salie R, Strijdom H, Lochner A. ANG II type I receptor antagonism improved nitric oxide production and enhanced eNOS and PKB/Akt expression in hearts from a rat model of insulin resistance. Mol Cell Biochem. 2011; 349:21–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kidney transplantation and ischemic conditioning: past, present and future perspectives
- Attenuation of Reperfusion Injury with Angiotensin AT1 Receptor Blockade in Rat Myocardial Ischemic Mbdel
- Influence of the Angiotensin II AT1 Receptor Antagonist on Reperfusion Injury in Rat Myocardial Ischemia Model
- Differential brain angiotensin-II type I receptor expression in hypertensive rats
- Effect of brain angiotensin II receptor antagonists and antisense oligonucleotide on drinking and renal renin in rats