J Clin Neurol.
2017 Apr;13(2):138-143. 10.3988/jcn.2017.13.2.138.
Neuroprotective Effect of Lacosamide on Hypoxic-Ischemic Brain Injury in Neonatal Rats
- Affiliations
-
- 1Department of Pediatrics, Korea University College of Medicine, Seoul, Korea. bleun@korea.ac.kr
- KMID: 2376014
- DOI: http://doi.org/10.3988/jcn.2017.13.2.138
Abstract
- BACKGROUND AND PURPOSE
Lacosamide (LCM) is an antiepileptic drug that enhances the slow inactivation of sodium channels and modulates collapsin response mediator protein-2. LCM was recently demonstrated to exert a neuroprotective effect in a murine model of traumatic brain injury and status epilepticus. Assuming the same underlying excitotoxicity-related brain injury mechanism, we hypothesized that LCM would have a neuroprotective effect in hypoxic-ischemic brain injury.
METHODS
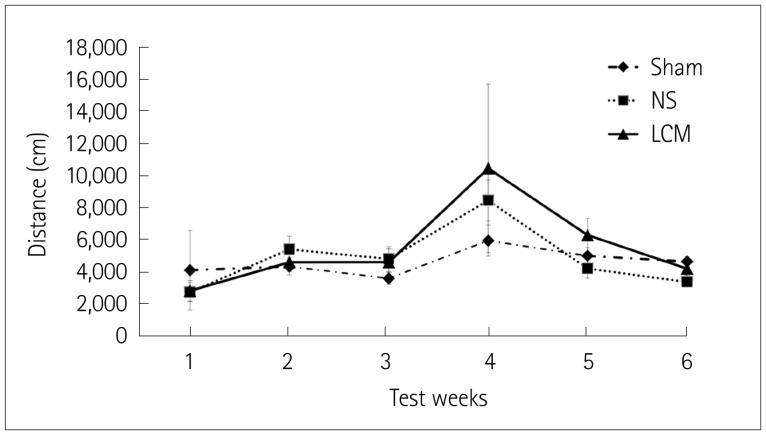
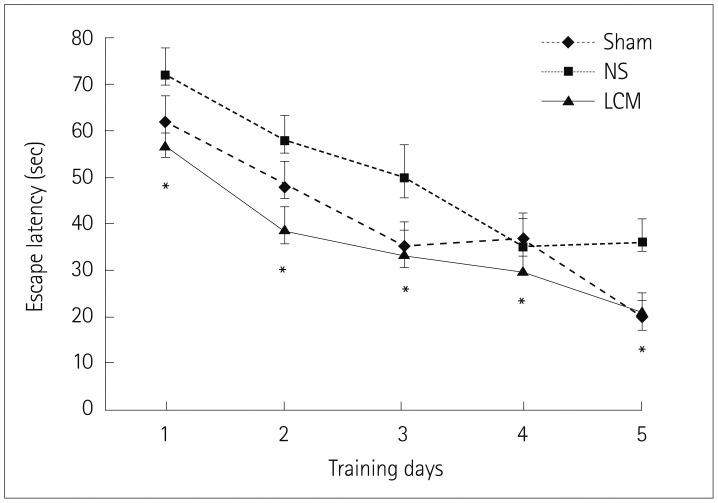
We divided rats into three groups at each testing session: pre- or postfed with LCM, fed with normal saline, and sham. A hypoxic-ischemic brain injury was induced by subjecting 7-day-old rats to right carotid artery coagulation followed by 2.5 h of exposure to 8% oxygen. The animals were killed on postnatal day 12 to evaluate the severity of brain damage. Open field testing was also performed between week 2 and week 6, and the Morris water maze test was performed in week 7 after hypoxia-ischemia.
RESULTS
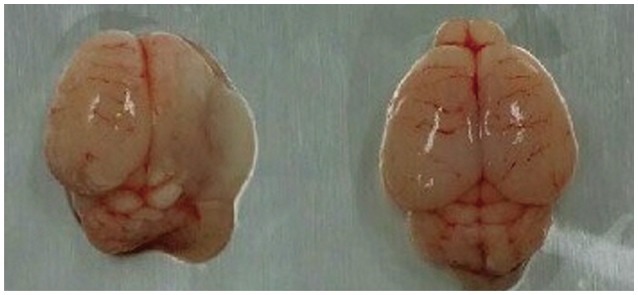
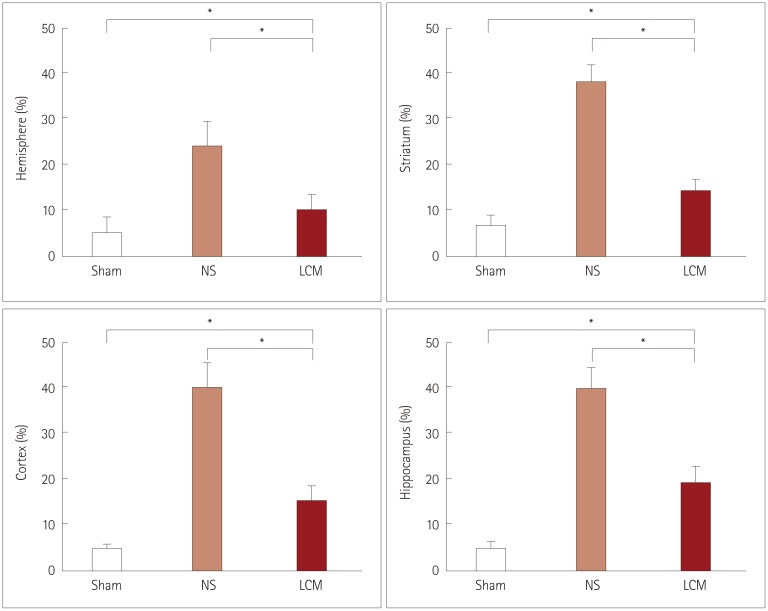
The incidence of liquefactive cerebral infarction was lower in rats prefed with LCM at 100 mg/kg/dose, with the mortality rate being higher at higher doses (200 and 300 mg/kg/dose). The infarct areas were smaller in LCM-prefed rats in several brain regions including the hemisphere, hippocampus, cortex, and striatum. Spatial learning and memory function were better in LCM-prefed rats (p<0.05). No effect was observed in postfed rats.
CONCLUSIONS
This study suggests that LCM pretreatment exerts a neuroprotective effect on hypoxia-ischemia in neonatal rats. The obtained results suggest that LCM pretreatment could be used as an effective neuroprotective method for neonates under hypoxic-ischemic conditions including heart surgery.
Keyword
MeSH Terms
-
Animals
Brain Injuries*
Brain*
Carotid Arteries
Cerebral Infarction
Hippocampus
Humans
Incidence
Infant, Newborn
Memory
Methods
Mortality
Neuroprotection
Neuroprotective Agents*
Oxygen
Rats*
Semaphorin-3A
Sodium Channels
Spatial Learning
Status Epilepticus
Thoracic Surgery
Water
Neuroprotective Agents
Oxygen
Semaphorin-3A
Sodium Channels
Water
Figure
Reference
-
1. Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Ann Neurol. 2003; 53:693–702. PMID: 12783414.
Article2. Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004; 35:1460–1465. PMID: 15105511.
Article3. Noh MR, Kim SK, Sun W, Park SK, Choi HC, Lim JH, et al. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Exp Neurol. 2006; 201:470–478. PMID: 16884714.
Article4. Schubert S, Brandl U, Brodhun M, Ulrich C, Spaltmann J, Fiedler N, et al. Neuroprotective effects of topiramate after hypoxia-ischemia in newborn piglets. Brain Res. 2005; 1058:129–136. PMID: 16139822.
Article5. Licko T, Seeger N, Zellinger C, Russmann V, Matagne A, Potschka H. Lacosamide treatment following status epilepticus attenuates neuronal cell loss and alterations in hippocampal neurogenesis in a rat electrical status epilepticus model. Epilepsia. 2013; 54:1176–1185. PMID: 23614482.
Article6. Wang B, Dawson H, Wang H, Kernagis D, Kolls BJ, Yao L, et al. Lacosamide improves outcome in a murine model of traumatic brain injury. Neurocrit Care. 2013; 19:125–134. PMID: 23269559.
Article7. Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003; 34:325–337. PMID: 12909079.
Article8. Calabresi P, Centonze D, Bernardi G. Cellular factors controlling neuronal vulnerability in the brain: a lesson from the striatum. Neurology. 2000; 55:1249–1255. PMID: 11092223.
Article9. Meldrum BS. Update on the mechanism of action of antiepileptic drugs. Epilepsia. 1996; 37(Suppl 6):S4–S11.
Article10. Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004; 118:687–698. PMID: 15369669.11. Vartanian MG, Cordon JJ, Kupina NC, Schielke GP, Posner A, Raser KJ, et al. Phenytoin pretreatment prevents hypoxic-ischemic brain damage in neonatal rats. Brain Res Dev Brain Res. 1996; 95:169–175. PMID: 8874891.
Article12. Chan SA, Reid KH, Schurr A, Miller JJ, Iyer V, Tseng MT. Fosphenytoin reduces hippocampal neuronal damage in rat following transient global ischemia. Acta Neurochir (Wien). 1998; 140:175–180. PMID: 10398998.
Article13. Edmonds HL Jr, Jiang YD, Zhang PY, Shank R. Topiramate as a neuroprotectant in a rat model of global ischemia-induced neurodegeneration. Life Sci. 2001; 69:2265–2277. PMID: 11669469.
Article14. Yang Y, Shuaib A, Li Q, Siddiqui MM. Neuroprotection by delayed administration of topiramate in a rat model of middle cerebral artery embolization. Brain Res. 1998; 804:169–176. PMID: 9757028.
Article15. Owen AJ, Ijaz S, Miyashita H, Wishart T, Howlett W, Shuaib A. Zonisamide as a neuroprotective agent in an adult gerbil model of global forebrain ischemia: a histological, in vivo microdialysis and behavioral study. Brain Res. 1997; 770:115–122. PMID: 9372210.
Article16. Wiard RP, Dickerson MC, Beek O, Norton R, Cooper BR. Neuroprotective properties of the novel antiepileptic lamotrigine in a gerbil model of global cerebral ischemia. Stroke. 1995; 26:466–472. PMID: 7886726.
Article17. Hanon E, Klitgaard H. Neuroprotective properties of the novel antiepileptic drug levetiracetam in the rat middle cerebral artery occlusion model of focal cerebral ischemia. Seizure. 2001; 10:287–293. PMID: 11466025.
Article18. Beydoun A, D'Souza J, Hebert D, Doty P. Lacosamide: pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother. 2009; 9:33–42. PMID: 19102666.
Article19. Beyreuther BK, Freitag J, Heers C, Krebsfänger N, Scharfenecker U, Stöhr T. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007; 13:21–42. PMID: 17461888.
Article20. Errington AC, Stöhr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltagegated sodium channels. Mol Pharmacol. 2008; 73:157–169. PMID: 17940193.
Article21. Halford JJ, Lapointe M. Clinical perspectives on lacosamide. Epilepsy Curr. 2009; 9:1–9. PMID: 19396339.
Article22. Patsalos PN, Berry DJ. Pharmacotherapy of the third-generation AEDs: lacosamide, retigabine and eslicarbazepine acetate. Expert Opin Pharmacother. 2012; 13:699–715. PMID: 22404663.
Article23. Wilson SM, Xiong W, Wang Y, Ping X, Head JD, Brittain JM, et al. Prevention of posttraumatic axon sprouting by blocking collapsin response mediator protein 2-mediated neurite outgrowth and tubulin polymerization. Neuroscience. 2012; 210:451–466. PMID: 22433297.
Article24. Hensley K, Venkova K, Christov A, Gunning W, Park J. Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol. 2011; 43:180–191. PMID: 21271304.
Article25. Touma E, Kato S, Fukui K, Koike T. Calpain-mediated cleavage of collapsin response mediator protein(CRMP)-2 during neurite degeneration in mice. Eur J Neurosci. 2007; 26:3368–3381. PMID: 18052987.
Article26. Thomas D, Scharfenecker U, Schiltmeyer B, Doty P, Cawello W, Horstmann R. Low potential for drug-drug-interaction of lacosamide. Epilepsia. 2006; 47(Suppl 4):200.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effect of Growth Hormone in Neonatal Rat with Hypoxic Ischemic Brain Injury
- The effect of erythropoietin in neonatal rat model of hypoxic-ischemic brain injury
- Neuroprotective effects of resveratrol via anti-apoptosis on hypoxic-ischemic brain injury in neonatal rats
- Effect of the Heme Oxygenase Inhibitor on the Hypoxic Ischemic Brain Injury in the Neonatal Rat
- Possible Neuroprotective Role of Exogenous Growth Hormone on Hypoxic-Ischemic Brain Injury in Neonatal Rats