J Breast Cancer.
2013 Mar;16(1):60-65.
Male Breast Cancer: 37-Year Data Study at a Single Experience Center in Turkey
- Affiliations
-
- 1Department of Medical Oncology, Cerrahpasa Medical School, Istanbul, Turkey. deniztural@gmail.com
- 2Department of Surgery, Cerrahpasa Medical School, Istanbul, Turkey.
- 3Department of Radiation Oncology, Cerrahpasa Medical School, Istanbul, Turkey.
Abstract
- PURPOSE
The aim of this study is to evaluate the effects of prognostic factors on the overall survival (OS) and locoregional control (LC) among male breast cancer (MBC) patients treated at Cerrahpasa Medical School Hospital, along with a review of the related literature.
METHODS
The data of 86 patients treated for MBC from 1973 to 2010 are retrospectively reviewed. Patient demographics and clinical information, including the date of diagnosis, treatment, clinical course, and the date and causes of death are routinely recorded.
RESULTS
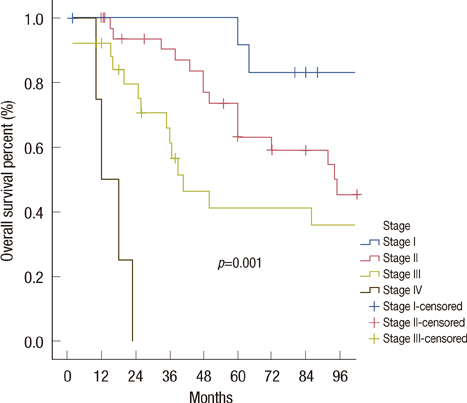
Median follow-up was 66 months. Isolated local-regional recurrence and distant metastases were observed in 15 (17.4%) and 24 (34.1%) of the cases, respectively. The 5-year OS rate was 65.8%; the disease-free survival rate was 72.4%, and the LC rate was 89.7%. The prognostic factors influencing local relapse were the T stage (p=0.002) and the chest wall muscular invasion (p=0.027) in the univariate analysis. The prognostic factors influencing OS were the presence of a positive axillary lymph node (p=0.001) and the T stage (p=0.001) in the univariate analysis. The T stage (p=0.008) and node (N) stage (p=0.038) were significant prognostic factors for OS in the multivariate analyses. Also, the T stage (p=0.034) was found to be significant for LC.
CONCLUSION
We found that only the tumor size and lymph node status were independent prognostic factors for survival. In addition, only the tumor size was an independent prognostic factor for locoregional relapse. Modified radical mastectomy and conservative surgical procedures had similar outcomes for LC.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.
Article2. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004. 101:51–57.3. Margaria E, Chiusa L, Ferrari L, Dal Canton O, Pich A. Therapy and survival in male breast carcinoma: a retrospective analysis of 50 cases. Oncol Rep. 2000. 7:1035–1039.
Article4. Evans GF, Anthony T, Turnage RH, Schumpert TD, Levy KR, Amirkhan RH, et al. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am J Surg. 2001. 181:96–100.
Article5. Spence RA, MacKenzie G, Anderson JR, Lyons AR, Bell M. Long-term survival following cancer of the male breast in Northern Ireland. A report of 81 cases. Cancer. 1985. 55:648–652.
Article6. Erlichman C, Murphy KC, Elhakim T. Male breast cancer: a 13-year review of 89 patients. J Clin Oncol. 1984. 2:903–909.
Article7. Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005. 10:471–479.
Article8. Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005. 104:2359–2364.
Article9. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.
Article10. Scheike O. Male breast cancer. 5. Clinical manifestations in 257 cases in Denmark. Br J Cancer. 1973. 28:552–561.11. Adami HO, Holmberg L, Malker B, Ries L. Long-term survival in 406 males with breast cancer. Br J Cancer. 1985. 52:99–103.
Article12. Gennari R, Curigliano G, Jereczek-Fossa BA, Zurrida S, Renne G, Intra M, et al. Male breast cancer: a special therapeutic problem. Anything new? (Review). Int J Oncol. 2004. 24:663–670.
Article13. Heinig J, Jackisch C, Rody A, Koch O, Buechter D, Schneider HP. Clinical management of breast cancer in males: a report of four cases. Eur J Obstet Gynecol Reprod Biol. 2002. 102:67–73.
Article14. Morimoto T, Komaki K, Yamakawa T, Tanaka T, Oomine Y, Konishi Y, et al. Cancer of the male breast. J Surg Oncol. 1990. 44:180–184.
Article15. Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002. 137:678–687.
Article16. Olsson H. Estrogen receptor content in malignant breast tumors in men: a review. J Mammary Gland Biol Neoplasia. 2000. 5:283–287.17. Stalsberg H, Thomas DB, Rosenblatt KA, Jimenez LM, McTiernan A, Stemhagen A, et al. Histologic types and hormone receptors in breast cancer in men: a population-based study in 282 United States men. Cancer Causes Control. 1993. 4:143–151.
Article18. Muir D, Kanthan R, Kanthan SC. Male versus female breast cancers. A population-based comparative immunohistochemical analysis. Arch Pathol Lab Med. 2003. 127:36–41.19. Bloom KJ, Govil H, Gattuso P, Reddy V, Francescatti D. Status of HER-2 in male and female breast carcinoma. Am J Surg. 2001. 182:389–392.
Article20. Curigliano G, Colleoni M, Renne G, Mazzarol G, Gennari R, Peruzzotti G, et al. Recognizing features that are dissimilar in male and female breast cancer: expression of p21Waf1 and p27Kip1 using an immunohistochemical assay. Ann Oncol. 2002. 13:895–902.
Article21. Contractor KB, Kaur K, Rodrigues GS, Kulkarni DM, Singhal H. Male breast cancer: is the scenario changing. World J Surg Oncol. 2008. 6:58.
Article22. Salvadori B, Saccozzi R, Manzari A, Andreola S, Conti RA, Cusumano F, et al. Prognosis of breast cancer in males: an analysis of 170 cases. Eur J Cancer. 1994. 30A:930–935.
Article23. Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005. 103:2006–2014.
Article24. Kwiatkowska E, Teresiak M, Filas V, Karczewska A, Breborowicz D, Mackiewicz A. BRCA2 mutations and androgen receptor expression as independent predictors of outcome of male breast cancer patients. Clin Cancer Res. 2003. 9:4452–4459.25. Joshi MG, Lee AK, Loda M, Camus MG, Pedersen C, Heatley GJ, et al. Male breast carcinoma: an evaluation of prognostic factors contributing to a poorer outcome. Cancer. 1996. 77:490–498.
Article26. Willsher PC, Leach IH, Ellis IO, Bell JA, Elston CW, Bourke JB, et al. Male breast cancer: pathological and immunohistochemical features. Anticancer Res. 1997. 17(3C):2335–2338.27. Perkins GH, Middleton LP, Garcia SM, Strom EA, McNeese MD, Kuerer HM, et al. Male breast carcinoma: outcomes and predictors of localregional failure in patients treated without radiation therapy. Breast Cancer Res Treat. 2002. 76:Suppl 1. S121.28. Chakravarthy A, Kim CR. Post-mastectomy radiation in male breast cancer. Radiother Oncol. 2002. 65:99–103.
Article29. Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010. 28:2114–2122.
Article30. Wernberg JA, Yap J, Murekeyisoni C, Mashtare T, Wilding GE, Kulkarni SA. Multiple primary tumors in men with breast cancer diagnoses: a SEER database review. J Surg Oncol. 2009. 99:16–19.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Features of the Male Breast Cancer-13 Cases
- Neutrophil to Lymphocyte Ratio May Predict Mortality in Breast Cancer Patients
- If Progesterone Is Blamed for Breast Cancer Development, Why Are We Still Using Tamoxifen?
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€