Yonsei Med J.
2016 May;57(3):790-794. 10.3349/ymj.2016.57.3.790.
Simple Purification of Adeno-Associated Virus-DJ for Liver-Specific Gene Expression
- Affiliations
-
- 1Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, USA. yamoon15@inha.ac.kr
- 2Department of Molecular Medicine, Inha University School of Medicine, Incheon, Korea.
- KMID: 2374103
- DOI: http://doi.org/10.3349/ymj.2016.57.3.790
Abstract
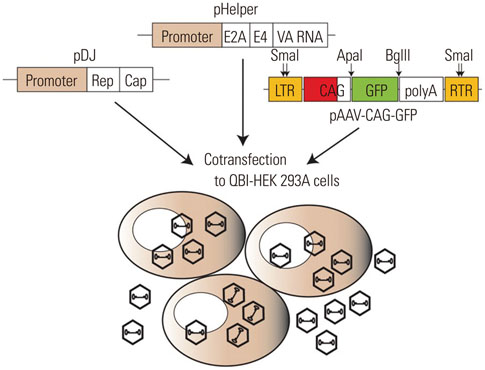
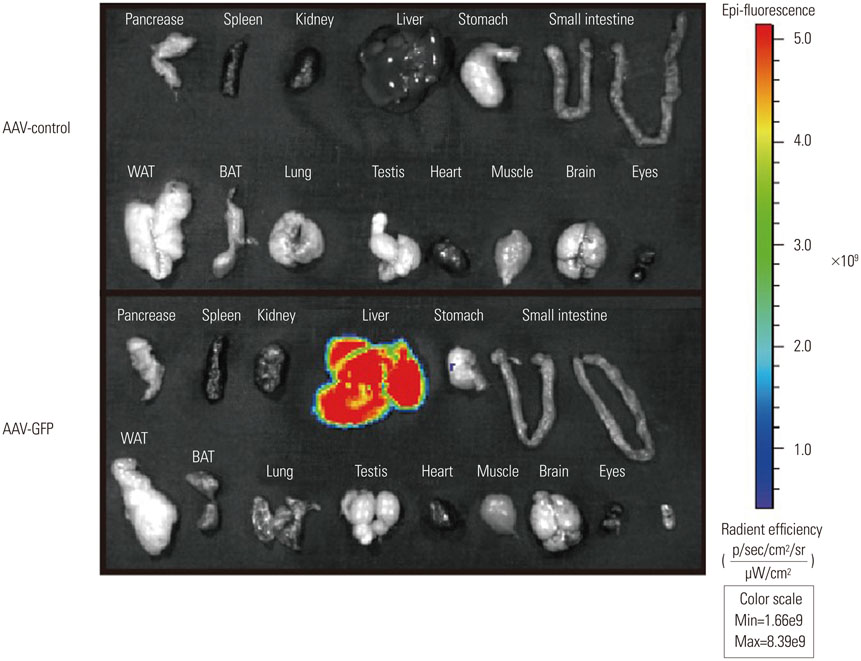
- Recombinant gene expression using adeno-associated viruses (AAVs) has become a valuable tool in animal studies, as they mediate safe expression of transduced genes for several months. The liver is a major organ of metabolism, and liver-specific expression of a gene can be an invaluable tool for metabolic studies. AAV-DJ is a recombinant AAV generated by the gene shuffling of various AAV serotypes and shares characteristics of AAV2 and AAV8. AAV-DJ contains a heparin-binding domain in its capsid, which suggests that a heparin column could be used for the purification of the AAV. Given that AAV-DJ has been only recently available, relatively little is known about the optimal preparation/purification and application of AAV-DJ. Here, we present a simple large-scale preparation method that can generate 3×10(13) viral particles for in vivo experiments and demonstrate liver-specific gene expression via systemic injection in mice.
MeSH Terms
Figure
Reference
-
1. Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993; 90:2812–2816.
Article2. Becker TC, Noel RJ, Coats WS, Gómez-Foix AM, Alam T, Gerard RD, et al. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994; 43(Pt A):161–189.3. Konz JO, Lee AL, Lewis JA, Sagar SL. Development of a purification process for adenovirus: controlling virus aggregation to improve the clearance of host cell DNA. Biotechnol Prog. 2005; 21:466–472.
Article4. Yang Y, Nunes FA, Berencsi K, Furth EE, Gönczöl E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994; 91:4407–4411.
Article5. Kozarsky KF, Wilson JM. Gene therapy of hypercholesterolemic disorders. Trends Cardiovasc Med. 1995; 5:205–209.
Article6. Jooss K, Turka LA, Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998; 5:309–319.
Article7. Mandel RJ, Rendahl KG, Spratt SK, Snyder RO, Cohen LK, Leff SE. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson's disease. J Neurosci. 1998; 18:4271–4284.
Article8. Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000; 24:257–261.
Article9. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011; 365:2357–2365.
Article10. Nakai H, Thomas CE, Storm TA, Fuess S, Powell S, Wright JF, et al. A limited number of transducible hepatocytes restricts a wide-range linear vector dose response in recombinant adeno-associated virus-mediated liver transduction. J Virol. 2002; 76:11343–11349.
Article11. Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006; 1:1412–1428.
Article12. Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006; 12:342–347.
Article13. Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, et al. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther. 2006; 13:77–87.
Article14. Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005; 79:214–224.
Article15. Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005; 105:3079–3086.
Article16. Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006; 14:45–53.
Article17. Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003; 102:2412–2419.
Article18. Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008; 82:5887–5911.
Article19. Lerch TF, O'Donnell JK, Meyer NL, Xie Q, Taylor KA, Stagg SM, et al. Structure of AAV-DJ, a retargeted gene therapy vector: cryo-electron microscopy at 4.5 Å resolution. Structure. 2012; 20:1310–1320.
Article20. Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002; 277:9520–9528.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant AAV Vector with MITF-M Promoter for Melanoma Gene Therapy
- Human Pluripotent Stem Cell-Derived Retinal Organoids: A Viable Platform for Investigating the Efficacy of Adeno-Associated Virus Gene Therapy
- Imaging of Herpes Simplex Virus Type 1 Thymidine Kinase Gene Expression with Radiolabeled 5-(2-iodovinyl)-2'-deoxyuridine (IVDU) in Liver by Hydrodynamic-based Procedure
- Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo
- Development of Trans Attack Anticancer Gene Vaccine Interact in HPV Oncoprotein Expression Inhibition