J Korean Med Sci.
2016 Jul;31(7):1075-1081. 10.3346/jkms.2016.31.7.1075.
Management of Suspicious Mucosa-Associated Lymphoid Tissue Lymphoma in Gastric Biopsy Specimens Obtained during Screening Endoscopy
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine and Gastrointestinal Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Internal Medicine and Healthcare Research Institute, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul, Korea. mdchlee@gmail.com
- 3Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2373726
- DOI: http://doi.org/10.3346/jkms.2016.31.7.1075
Abstract
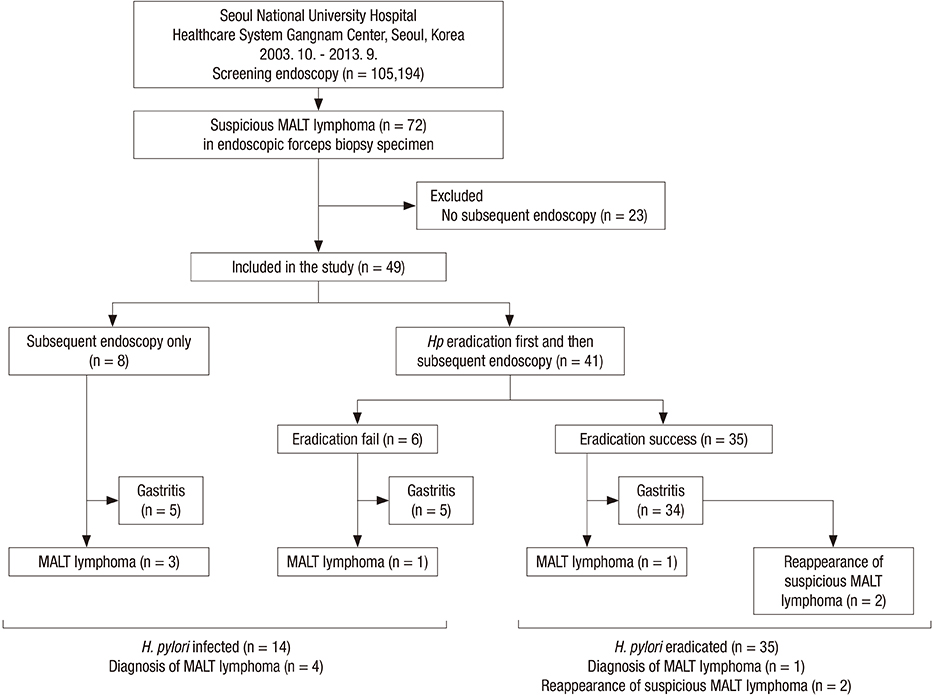
- It is often difficult to differentiate gastric mucosa-associated lymphoid tissue (MALT) lymphoma from Helicobacter pylori-associated follicular gastritis, and thus, it becomes unclear how to manage these diseases. This study aimed to explore the management strategy for and the long-term outcomes of suspicious gastric MALT lymphoma detected by forceps biopsy during screening upper endoscopy. Between October 2003 and May 2013, consecutive subjects who were diagnosed with suspicious gastric MALT lymphomas by screening endoscopy in a health checkup program in Korea were retrospectively enrolled. Suspicious MALT lymphoma was defined as a Wotherspoon score of 3 or 4 upon pathological evaluation of the biopsy specimen. Of 105,164 subjects who underwent screening endoscopies, 49 patients with suspicious MALT lymphomas who underwent subsequent endoscopy were enrolled. Eight patients received a subsequent endoscopy without H. pylori eradication (subsequent endoscopy only group), and 41 patients received H. pylori eradication first followed by endoscopy (eradication first group). MALT lymphoma development was significantly lower in the eradication first group (2/41, 4.9%) than in the subsequent endoscopy only group (3/8, 37.5%, P = 0.026). Notably, among 35 patients with successful H. pylori eradication, there was only one MALT lymphoma patient (2.9%) in whom complete remission was achieved, and there was no recurrence during a median 45 months of endoscopic follow-up. H. pylori eradication with subsequent endoscopy would be a practical management option for suspicious MALT lymphoma detected in a forceps biopsy specimen obtained during screening upper endoscopy.
Keyword
MeSH Terms
-
Adult
Aged
Anti-Bacterial Agents/therapeutic use
Biopsy
Female
Follow-Up Studies
Gastric Mucosa/*pathology
Gastritis/diagnosis/etiology/microbiology
Gastroscopy
Helicobacter Infections/complications/*diagnosis/drug therapy
Humans
Lymphoma, B-Cell, Marginal Zone/complications/*diagnosis/pathology
Male
Middle Aged
Republic of Korea
Retrospective Studies
Anti-Bacterial Agents
Figure
Reference
-
1. Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007; 136:521–538.2. Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A. Gastric MALT lymphoma: old and new insights. Ann Gastroenterol. 2014; 27:27–33.3. Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori . Lancet. 1993; 342:575–577.4. Achyut BR, Moorchung N, Srivastava AN, Gupta NK, Mittal B. Risk of lymphoid follicle development in patients with chronic antral gastritis: role of endoscopic features, histopathological parameters, CagA status and interleukin-1 gene polymorphisms. Inflamm Res. 2008; 57:51–56.5. Doglioni C, Ponzoni M, Ferreri AJ, Savio A. Gruppo Italiano Patologi Apparato Digerente (GIPAD); Società Italiana di Anatomia Patologica e Citopatologia Diagnostica/International Academy of Pathology, Italian division (SIAPEC/IAP). Gastric lymphoma: the histology report. Dig Liver Dis. 2011; 43:Suppl 4. S310–8.6. Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011; 60:747–758.7. Zucca E, Dreyling M; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21:Suppl 5. v175–6.8. Hummel M, Oeschger S, Barth TF, Loddenkemper C, Cogliatti SB, Marx A, Wacker HH, Feller AC, Bernd HW, Hansmann ML, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut. 2006; 55:782–787.9. Wündisch T, Neubauer A, Stolte M, Ritter M, Thiede C. B-cell monoclonality is associated with lymphoid follicles in gastritis. Am J Surg Pathol. 2003; 27:882–887.10. Xu W, Zhou C, Zhang G, Wang H, Wang L, Guo J. Repeating gastric biopsy for accuracy of gastric lymphoma diagnosis. Gastroenterol Nurs. 2010; 33:313–317.11. Taal BG, Boot H, van Heerde P, de Jong D, Hart AA, Burgers JM. Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept. Gut. 1996; 39:556–561.12. Stolte M. Helicobacter pylori gastritis and gastric MALT-lymphoma. Lancet. 1992; 339:745–746.13. Rudolph B, Bayerdörffer E, Ritter M, Müller S, Thiede C, Neubauer B, Lehn N, Seifert E, Otto P, Hatz R, et al. Is the polymerase chain reaction or cure of Helicobacter pylori infection of help in the differential diagnosis of early gastric mucosa-associated lymphatic tissue lymphoma? J Clin Oncol. 1997; 15:1104–1109.14. Chung SJ, Park MJ, Kang SJ, Kang HY, Chung GE, Kim SG, Jung HC. Effect of annual endoscopic screening on clinicopathologic characteristics and treatment modality of gastric cancer in a high-incidence region of Korea. Int J Cancer. 2012; 131:2376–2384.15. Choi KS, Jun JK, Lee HY, Park S, Jung KW, Han MA, Choi IJ, Park EC. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. 2011; 102:1559–1564.16. Nakamura T, Seto M, Tajika M, Kawai H, Yokoi T, Yatabe Y, Nakamura S. Clinical features and prognosis of gastric MALT lymphoma with special reference to responsiveness to H. pylori eradication and API2-MALT1 status. Am J Gastroenterol. 2008; 103:62–70.17. Kolve M, Fischbach W, Greiner A, Wilms K; German Gastrointestinal Lymphoma Study Group. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-Hodgkin’s lymphoma. Gastrointest Endosc. 1999; 49:307–315.18. Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.19. Musshoff K. Clinical staging classification of non-Hodgkin’s lymphomas (author’s transl). Strahlentherapie. 1977; 153:218–221.20. Shiozawa E, Norose T, Kaneko K, Yamochi-Onizuka T, Takimoto M, Imawari M, Ota H. Clinicopathological comparison of the World Health Organization/Wotherspoon score to the Groupe d’Etude des Lymphomes de l’Adult grade for the post-treatment evaluation of gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2009; 24:307–315.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Low-Grade Gastric Mucosa Associated Lymphoid Tissue Lymphoma with Normal Endoscopic Appearance
- Role of Chemotherapy in Gastric Marginal Zone B-Cell Lymphoma of Mucosa-Associated Lymphoid Tissue (MALT) Type
- Gastric Mucosa-associated Lymphoid Tissue Lymphoma Based on Outcome of Domestic Treatment
- Endoscopic features aiding the diagnosis of gastric mucosa-associated lymphoid tissue lymphoma
- A Case of Gastric MALT Lymphoma Presenting as Nodular Gastritis in a Child