Ann Lab Med.
2016 Mar;36(2):101-110. 10.3343/alm.2016.36.2.101.
Clinicopathological Implications of Mitochondrial Genome Alterations in Pediatric Acute Myeloid Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea. 98lani@gmail.com, mgshin@chonnam.ac.kr
- 2Department of Pediatrics, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Environmental Health Center for Childhood Leukemia and Cancer, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 4Brain Korea 21 Plus Project, Chonnam National University Medical School, Gwangju, Korea.
- 5College of Korean Medicine, Dongshin University, Naju, Korea, Korea.
- 6Department of Cell Therapy, Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany.
- KMID: 2373508
- DOI: http://doi.org/10.3343/alm.2016.36.2.101
Abstract
- BACKGROUND
To the best of our knowledge, the association between pediatric AML and mitochondrial aberrations has not been studied. We investigated various mitochondrial aberrations in pediatric AML and evaluated their impact on clinical outcomes.
METHODS
Sequencing, mitochondrial DNA (mtDNA) copy number determination, mtDNA 4,977-bp large deletion assessments, and gene scan analyses were performed on the bone marrow mononuclear cells of 55 pediatric AML patients and on the peripheral blood mononuclear cells of 55 normal controls. Changes in the mitochondrial mass, mitochondrial membrane potential, and intracellular reactive oxygen species (ROS) levels were also examined.
RESULTS
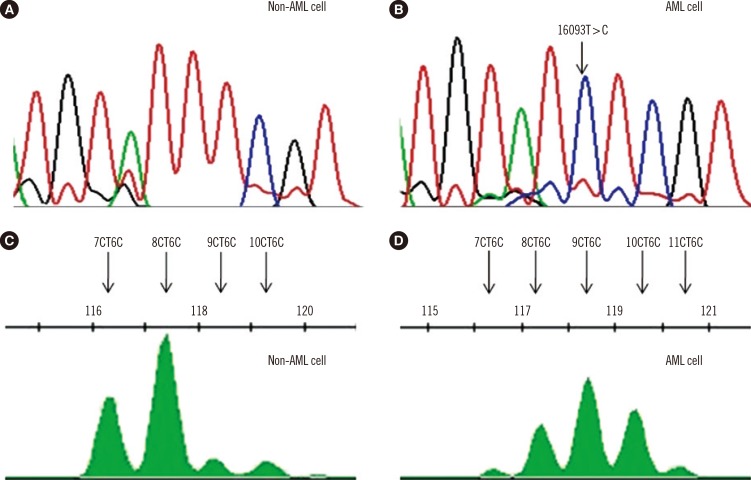
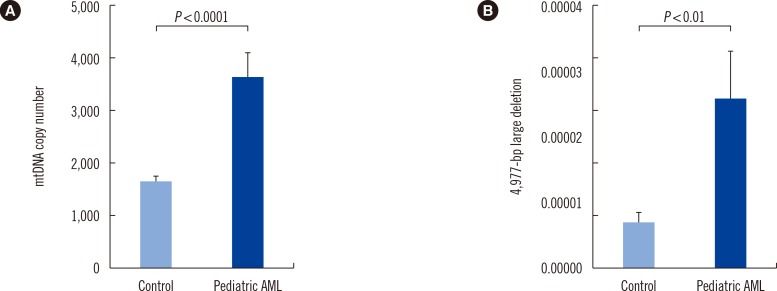
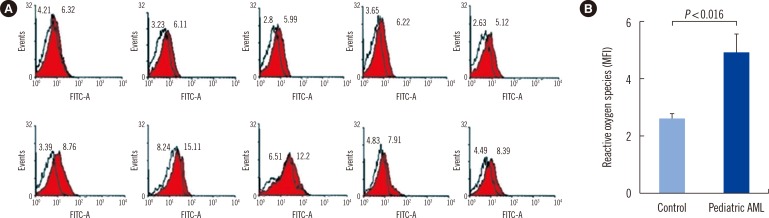
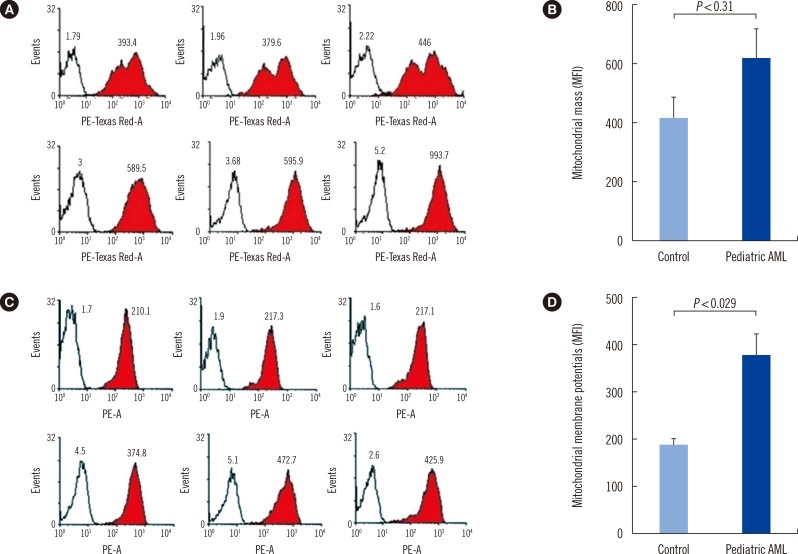
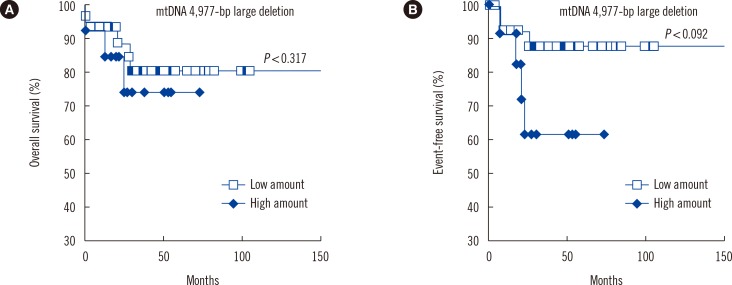
mtDNA copy numbers were about two-fold higher in pediatric AML cells than in controls (P<0.0001). Furthermore, a close relationship was found between mtDNA copy number tertiles and the risk of pediatric AML. Intracellular ROS levels, mitochondrial mass, and mitochondrial membrane potentials were all elevated in pediatric AML. The frequency of the mtDNA 4,977-bp large deletion was significantly higher (P< 0.01) in pediatric AML cells, and pediatric AML patients harboring high amount of mtDNA 4,977-bp deletions showed shorter overall survival and event-free survival rates, albeit without statistical significance.
CONCLUSIONS
The present findings demonstrate an association between mitochondrial genome alterations and the risk of pediatric AML.
MeSH Terms
-
Bone Marrow Cells/metabolism
Case-Control Studies
Child
Cohort Studies
DNA, Mitochondrial/chemistry/genetics/metabolism
Female
Flow Cytometry
Gene Deletion
Gene Dosage
*Genome, Mitochondrial
Humans
Leukemia, Myeloid, Acute/genetics/mortality/*pathology
Male
Membrane Potential, Mitochondrial
Minisatellite Repeats/genetics
Odds Ratio
Reactive Oxygen Species/metabolism
Sequence Analysis, DNA
Survival Rate
DNA, Mitochondrial
Reactive Oxygen Species
Figure
Reference
-
1. Chinnery PF, Schon EA. Mitochondria. J Neurol Neurosurg Psychiatry. 2003; 74:1188–1199. PMID: 12933917.
Article2. DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003; 348:2656–2668. PMID: 12826641.
Article3. Lim SW, Kim HR, Kim HY, Huh JW, Kim YJ, Shin JH, et al. High-frequency minisatellite instability of the mitochondrial genome in colorectal cancer tissue associated with clinicopathological values. Int J Cancer. 2012; 131:1332–1341. PMID: 22120612.
Article4. Park SY, Shin MG, Kim HR, Oh JY, Kim SH, Shin JH, et al. Alteration of mitochondrial DNA sequence and copy number in nasal polyp tissue. Mitochondrion. 2009; 9:318–325. PMID: 19426839.
Article5. Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981; 290:457–465. PMID: 7219534.
Article6. Shin MG, Kajigaya S, Tarnowka M, McCoy JP Jr, Levin BC, Young NS. Mitochondrial DNA sequence heterogeneity in circulating normal human CD34 cells and granulocytes. Blood. 2004; 103:4466–4477. PMID: 15016645.
Article7. Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA. 1988; 85:6465–6467. PMID: 3413108.
Article8. Ishikawa K, Hayashi J. A novel function of mtDNA: its involvement in metastasis. Ann N Y Acad Sci. 2010; 1201:40–43. PMID: 20649537.
Article9. Penta JS, Johnson F, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001; 488:119–133. PMID: 11344040.
Article10. Ishikawa K, Koshikawa N, Takenaga K, Nakada K, Hayashi J. Reversible regulation of metastasis by ROS-generating mtDNA mutations. Mitochondrion. 2008; 8:339–344. PMID: 18727959.
Article11. Shadel GS. Expression and maintenance of mitochondrial DNA: new insights into human disease pathology. Am J Pathol. 2008; 172:1445–1456. PMID: 18458094.12. Yin PH, Lee HC, Chau GY, Wu YT, Li SH, Lui WY, et al. Alteration of the copy number and deletion of mitochondrial DNA in human hepatocellular carcinoma. Br J Cancer. 2004; 90:2390–2396. PMID: 15150555.
Article13. Macmillan CJ, Shoubridge EA. Mitochondrial DNA depletion: prevalence in a pediatric population referred for neurologic evaluation. Pediatr Neurol. 1996; 14:203–210. PMID: 8736403.
Article14. Yu M, Zhou Y, Shi Y, Ning L, Yang Y, Wei X, et al. Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life. 2007; 59:450–457. PMID: 17654121.
Article15. Xing J, Chen M, Wood CG, Lin J, Spitz MR, Ma J, et al. Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst. 2008; 100:1104–1112. PMID: 18664653.
Article16. Yu M, Wan Y, Zou Q. Reduced mitochondrial DNA copy number in Chinese patients with osteosarcoma. Transl Res. 2013; 161:165–171. PMID: 23177796.
Article17. Lan Q, Lim U, Liu CS, Weinstein SJ, Chanock S, Bonner MR, et al. A prospective study of mitochondrial DNA copy number and risk of non-Hodgkin lymphoma. Blood. 2008; 112:4247–4249. PMID: 18711000.
Article18. Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012; 120:3187–3205. PMID: 22879540.
Article19. Rubnitz JE, Inaba H. Childhood acute myeloid leukaemia. Br J Haematol. 2012; 159:259–276. PMID: 22966788.
Article20. Kwok CS, Quah TC, Ariffin H, Tay SK, Yeoh AE. Mitochondrial D-loop polymorphisms and mitochondrial DNA content in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011; 33:e239–e244. PMID: 21646920.
Article21. Chalandon Y, Vischer S, Helg C, Chapuis B, Roosnek E. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Geneva experience. Leukemia. 2003; 17:228–231. PMID: 12529684.
Article22. Kim HR, Shin MG, Kim MJ, Shin JH, Suh SP, Ryang DW. Characteristics of mitochondrial DNA sequence polymorphisms and haplogroups in Korean population. Genes and Genomics. 2008; 30:121–126. http://210.101.116.28/W_files/kiss3/09001745_pv.pdf.23. Pfeiffer H, Steighner R, Fisher R, Mörnstad H, Yoon CL, Holland MM. Mitochondrial DNA extraction and typing from isolated dentin-experimental evaluation in a Korean population. Int J Legal Med. 1998; 111:309–313. PMID: 9826090.
Article24. Stoneking M. Hypervariable sites in the mtDNA control region are mutational hotspots. Am J Hum Genet. 2000; 67:1029–1032. PMID: 10968778.
Article25. Chang DD, Clayton DA. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci USA. 1985; 82:351–355. PMID: 2982153.
Article26. Shin MG, Levin BC, Kim HJ, Kim HR, Lee IK, Cho D, et al. Profiling of length heteroplasmies in the human mitochondrial DNA control regions from blood cells in the Korean population. Electrophoresis. 2006; 27:1331–1340. PMID: 16502464.
Article27. Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006; 125:1241–1252. PMID: 16814712.
Article28. Silkjaer T, Nørgaard JM, Aggerholm A, Ebbesen LH, Kjeldsen E, Hokland P, et al. Characterization and prognostic significance of mitochondrial DNA variations in acute myeloid leukemia. Eur J Haematol. 2013; 90:385–396. PMID: 23444869.
Article29. Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005; 37:822–834. PMID: 15694841.
Article30. Clayton DA, Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967; 216:652–657. PMID: 6082459.
Article31. Clayton DA, Vinograd J. Complex mitochondrial DNA in leukemic and normal human myeloid cells. Proc Natl Acad Sci USA. 1969; 62:1077–1084. PMID: 5256408.
Article32. Fang H, Shen L, Chen T, He J, Ding Z, Wei J, et al. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer. 2010; 10:421. PMID: 20704735.
Article33. James AM, Murphy MP. How mitochondrial damage affects cell function. J Biomed Sci. 2002; 9:475–487. PMID: 12372986.
Article34. Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009; 36:125–131. PMID: 19302968.
Article35. Tseng LM, Yin PH, Chi CW, Hsu CY, Wu CW, Lee LM, et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes Chromosomes Cancer. 2006; 45:629–638. PMID: 16568452.
Article36. Wang Y, Liu VW, Xue WC, Cheung AN, Ngan HY. Association of decreased mitochondrial DNA content with ovarian cancer progression. Br J Cancer. 2006; 95:1087–1091. PMID: 17047655.
Article37. Mizumachi T, Suzuki S, Naito A, Carcel-Trullols J, Evans TT, Spring PM, et al. Increased mitochondrial DNA induces acquired docetaxel resistance in head and neck cancer cells. Oncogene. 2008; 27:831–838. PMID: 17637738.
Article38. Carew JS, Nawrocki ST, Xu RH, Dunner K, McConkey DJ, Wierda WG, et al. Increased mitochondrial biogenesis in primary leukemia cells: the role of endogenous nitric oxide and impact on sensitivity to fludarabine. Leukemia. 2004; 18:1934–1940. PMID: 15483672.
Article39. Kusao I, Agsalda M, Troelstrup D, Villanueva N, Shiramizu B. Chemotoxicity recovery of mitochondria in nonXMLLink_XYZHodgkin lymphoma resulting in minimal residual disease. Pediatr Blood Cancer. 2008; 51:193–197. PMID: 18322926.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spectrum of mitochondrial genome instability and implication of mitochondrial haplogroups in Korean patients with acute myeloid leukemia
- Recent advances in the treatment of pediatric acute leukemia
- Current treatment for pediatric acute myeloid leukemia
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- A Pediatric Case of Acute Myeloid Leukemia with t(3;5)(q25;q34)