J Korean Ophthalmol Soc.
2017 Mar;58(3):296-304. 10.3341/jkos.2017.58.3.296.
Comparison of Choroidal Thickness Change between Ranibizumab and Aflibercept in Age-related Macular Degeneration: Six Month Results
- Affiliations
-
- 1Cheil Eye Hospital, Daegu, Korea. flowerchild03@hanmail.net
- KMID: 2371910
- DOI: http://doi.org/10.3341/jkos.2017.58.3.296
Abstract
- PURPOSE
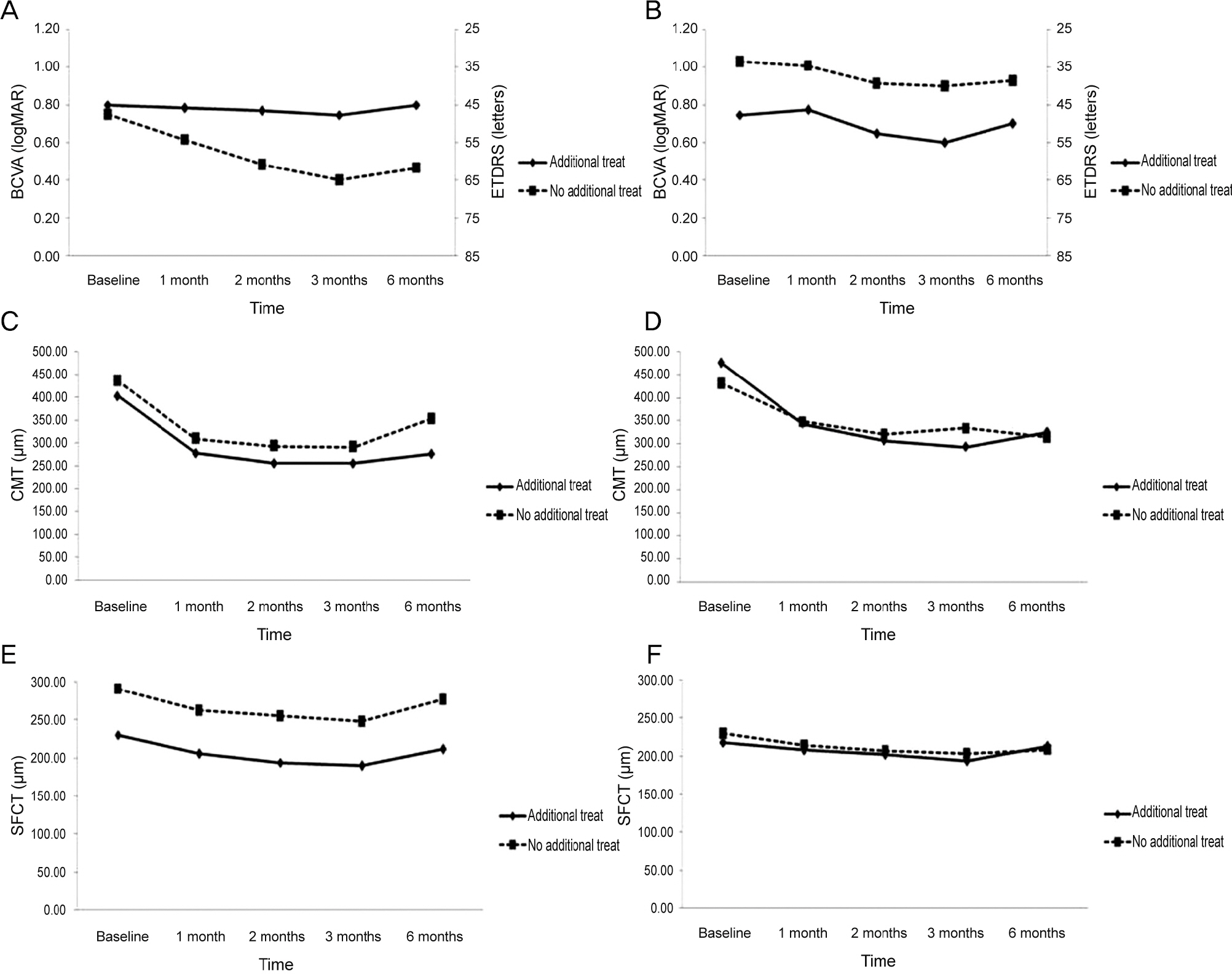
To compare the changes in subfoveal choroidal thickness between intravitreal aflibercept and ranibizumab injection in wet age-related macular degeneration (AMD).
METHODS
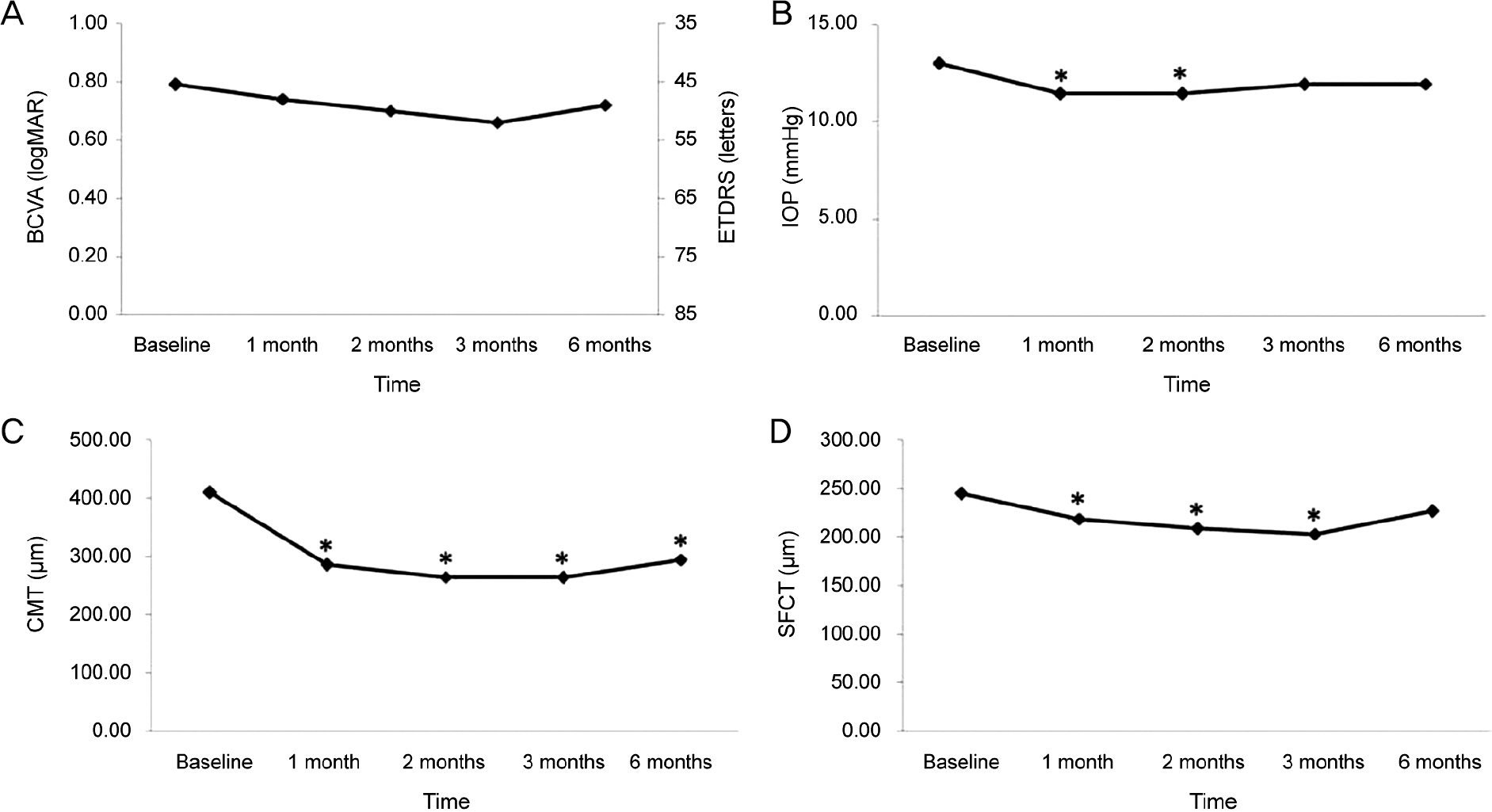
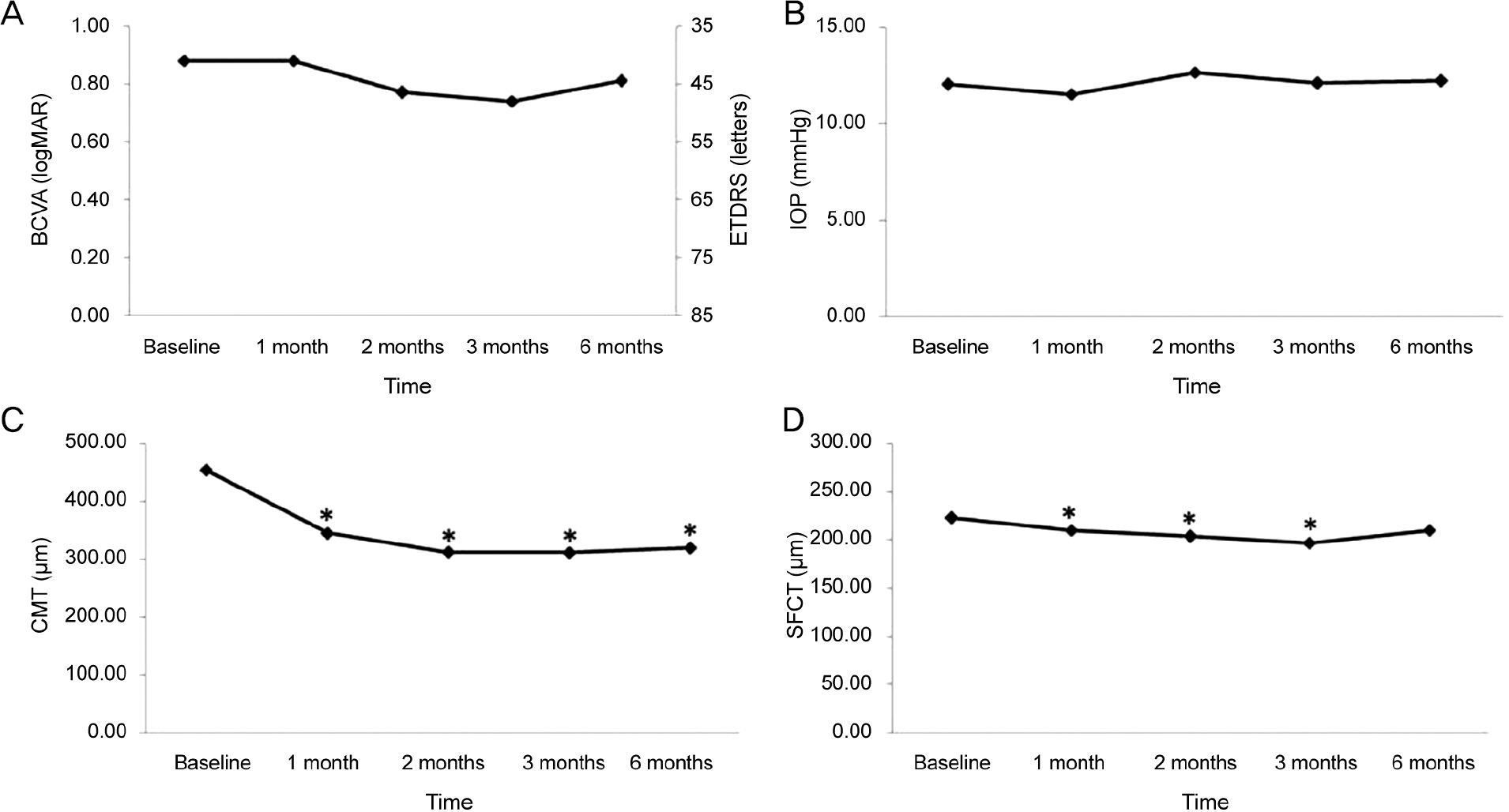
Fifty patients with wet AMD patients who were treated with aflibercpet or ranibizumab were evaluated retrospectively. All patients were treated with pro re nata after 3 consecutive monthly injections and were followed up for at least 6 months. We measured subfoveal choroidal thickness (SFCT) using enhanced depth imaging spectral-domain optical coherence tomography before the first injection and 1, 2, 3, and 6 months after initial injection.
RESULTS
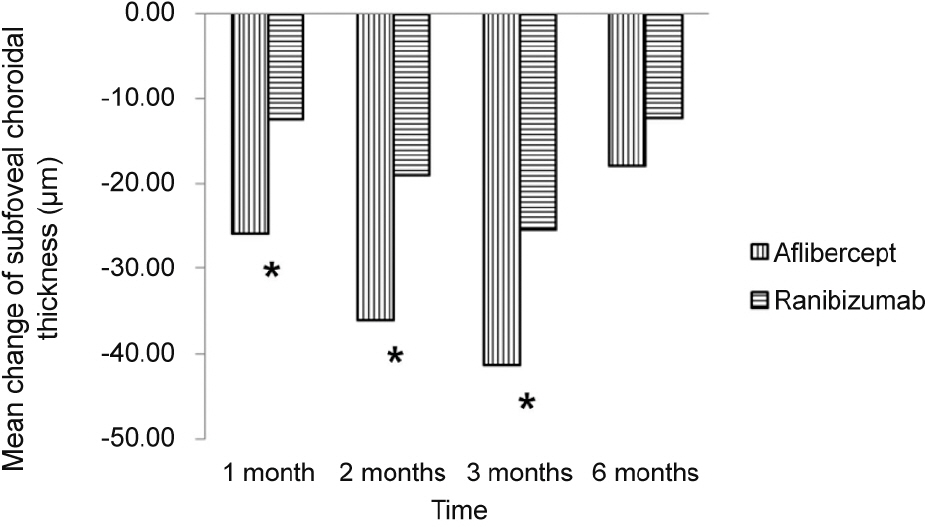
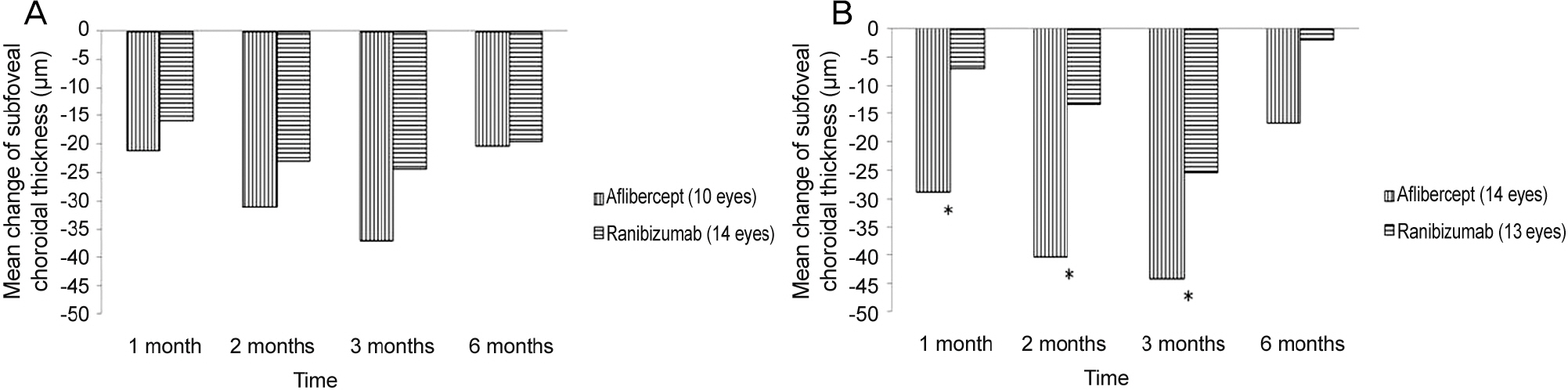
The SFCT measures before initial injection and 1, 2, 3, and 6 months after initial injection were 244.94 ± 103.77 µm, 219.04 ± 95.89 µm, 208.74 ± 91.03 µm, 203.64 ± 91.35 µm, and 226.98 ± 96.79 µm in the aflibercept group (25 eyes) and 222.68 ± 102.04 µm, 210.23 ± 95.91 µm, 203.66 ± 99.39 µm, 197.27 ± 100.25 µm, and 210.32 ± 111.86 µm in the ranibizumab group (28 eyes). Mean change in SFCT was greater in the aflibercept group at 1 month, 2 months, and 3 months after initial injection (p < 0.05), but there was no significant difference in the mean change in SFCT between the two groups at 6 months after initial injection (p > 0.05).
CONCLUSIONS
The decrease in SFCT was greater after aflibercept injection than ranibizumab injection in 3 consecutive months. However, at 6 months after initial injection, the difference in the change in SFCT was not significant.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Long-term Treatment Outcome of Intravitreal Aflibercept Monotherapy for Polypoidal Choroidal Vasculopathy
Ye Ji Kim, Sang Yun Han, Jong Woo Kim, Chul Gu Kim, Dong Won Lee, Jae Hui Kim
J Korean Ophthalmol Soc. 2018;59(3):238-245. doi: 10.3341/jkos.2018.59.3.238.
Reference
-
References
1. Augood C, Fletcher A, Bentham G. . Methods for a pop-ulation-based study of the prevalence of and risk factors for age-re-lated maculopathy and macular degeneration in elderly European populations: the EUREYE study. Ophthalmic Epidemiol. 2004; 11:117–29.
Article2. Friedman DS, O'Colmain BJ, Muñoz B. . Prevalence of age-re-lated macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.
Article3. Park SJ, Lee JH, Woo SJ. . Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–65.4. Rosenfeld PJ, Brown DM, Heier JS. . Ranibizumab for neo-vascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.
Article5. Brown DM, Kaiser PK, Michels M. . Ranibizumab versus ver-teporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
Article6. Avery RL, Pieramici DJ, Rabena MD. . Intravitreal bev-acizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.e5.
Article7. Folk JC, Stone EM. Ranibizumab therapy for neovascular age-re-lated macular degeneration. N Engl J Med. 2010; 363:1648–55.
Article8. Lanzetta P, Mitchell P, Wolf S, Veritti D. Different antivascular en-dothelial growth factor treatments and regimens and their out-comes in neovascular age-related macular degeneration: a liter-ature review. Br J Ophthalmol. 2013; 97:1497–507.
Article9. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG. . Ranibizumab and bevacizumab for treatment of neovascular age-re-lated macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–98.10. Heier JS, Brown DM, Chong V. . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.
Article11. Blaauwgeers HG, Holtkamp GM, Rutten H. . Polarized vas-cular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic para-crine relation. Am J Pathol. 1999; 155:421–8.
Article12. Saint-Geniez M, Kurihara T, Sekiyama E. . An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009; 106:18751–6.
Article13. Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal cho-roidal thickness after ranibizumab therapy for neovascular age-re-lated macular degeneration: 12-month results. Ophthalmology. 2012; 119:1621–7.
Article14. Koizumi H, Kano M, Yamamoto A. . Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol. 2015; 159:627–33.
Article15. Kim JH, Lee TG, Chang YS. . Short-term choroidal thickness changes in patients treated with either ranibizumab or aflibercept: a comparative study. Br J Ophthalmol. 2016; 100:1634–9.
Article16. Yun C, Oh J, Ahn J. . Comparison of intravitreal aflibercept and ranibizumab injections on subfoveal and peripapillary choroi-dal thickness in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1693–702.
Article17. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.
Article18. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 2008; 92:667–8.
Article19. Chappelow AV, Kaiser PK. Neovascular age-related macular de-generation: potential therapies. Drugs. 2008; 68:1029–36.20. Rudge JS, Thurston G, Davis S. . VEGF trap as a novel anti-angiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005; 70:411–8.21. Papadopoulos N, Martin J, Ruan Q. . Binding and neutraliza-tion of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–85.
Article22. Linsenmeier RA, Padnick-Silver L.Metabolic dependence of pho-toreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000; 41:3117–23.23. Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013; 37:68–89.
Article24. Julien S, Biesemeier A, Taubitz T, Schraermeyer U. Different ef-fects of intravitreally injected ranibizumab and aflibercept on reti-nal and choroidal tissues of monkey eyes. Br J Ophthalmol. 2014; 98:813–25.
Article25. Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010; 94:297–301.
Article26. Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term effi-cacy of intravitreal aflibercept in treatment-naive patients with pol-ypoidal choroidal vasculopathy. Retina. 2014; 34:2178–84.
Article27. Sasahara M, Tsujikawa A, Musashi K. . Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142:601–7.
Article28. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in pol-ypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–5.
Article29. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroi-dal thickness in normal, healthy subjects measured by spectral do-main optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:261–6.
Article30. Wei WB, Xu L, Jonas JB. . Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013; 120:175–80.
Article31. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–5.
Article32. Lee SW, Yu SY, Seo KH. . Diurnal variation in choroidal thick-ness in relation to sex, axial length, and baseline choroidal thick-ness in healthy Korean subjects. Retina. 2014; 34:385–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Full-thickness Macular Hole after Intravitreal Aflibercept Injection in a Patient with Wet Age-related Macular Degeneration
- Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Clinical Changes after Switching from Ranibizumab/Aflibercept to Bevacizumab in Exudative Age-related Macular Degeneration
- Comparison between Aflibercept, Ranibizumab Intravitreal Injection on Neovascular Age-related Macular Degeneration Patients