J Korean Ophthalmol Soc.
2017 Jan;58(1):62-68. 10.3341/jkos.2017.58.1.62.
Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Affiliations
-
- 1Department of Ophthalmology, Kim's Eye Hospital, Konyang University College of Medicine, Seoul, Korea. kjh7997@daum.net
- 2Department of Ophthalmology, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2367843
- DOI: http://doi.org/10.3341/jkos.2017.58.1.62
Abstract
- PURPOSE
To evaluate the efficacy of 3 bimonthly aflibercept injections for neovascular age-related macular degeneration (AMD) that showed limited response to 3 initial ranibizumab injections.
METHODS
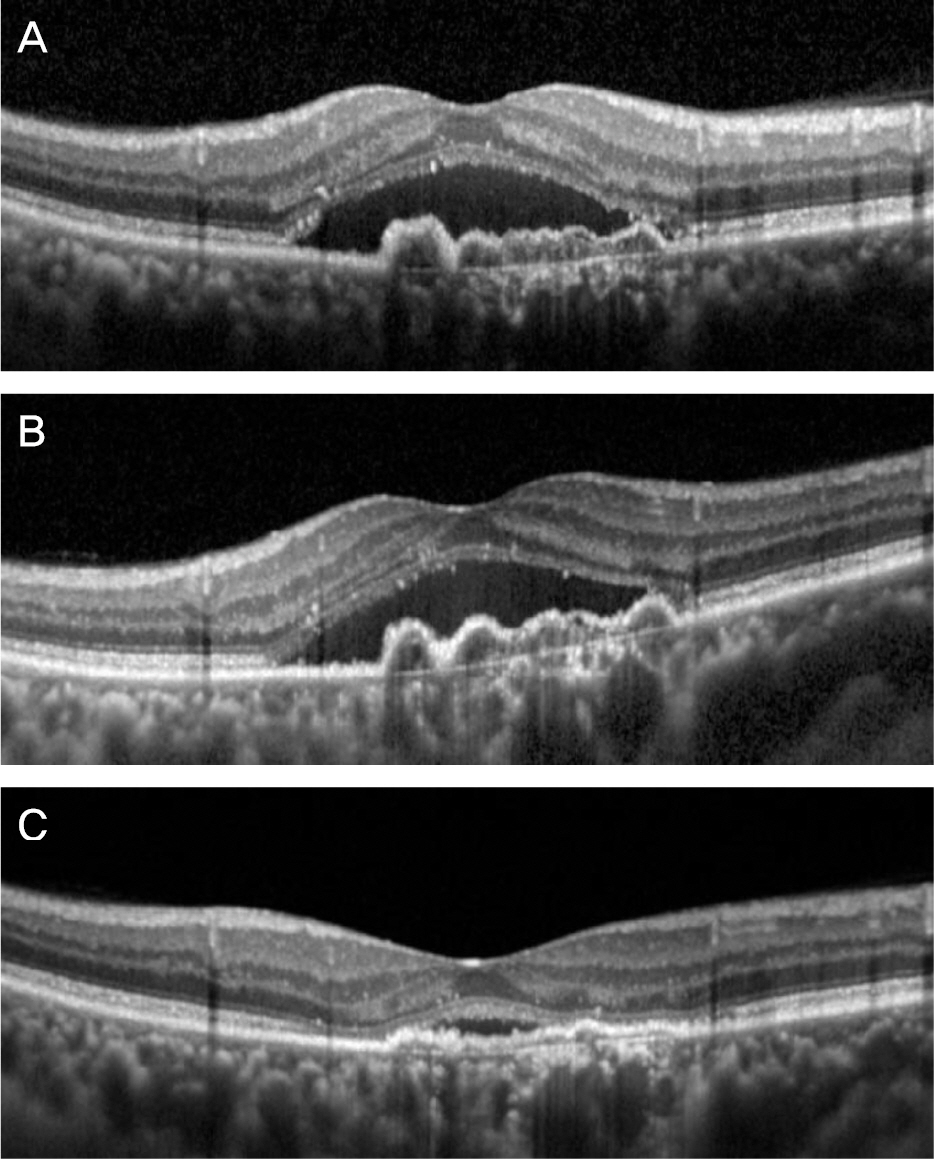
Three bimonthly aflibercept injections were performed for 21 eyes with neovascular AMD that was refractory to 3 monthly ranibizumab injections. Best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were measured at diagnosis, 1 month after 3 ranibizumab injections, and 1 month after 3 aflibercept injections, and these values were compared.
RESULTS
The mean logarithm of minimal angle of resolution (logMAR) BCVA at diagnosis, after ranibizumab therapy, and after aflibercept therapy was 0.62 ± 0.29, 0.73 ± 0.31, and 0.65 ± 0.28, respectively. The CRT at the aforementioned times was 427.0 ± 98.7 µm, 409.5 ± 78.7 µm, and 315.9 ± 98.2 µm, respectively. When compared with the value measured after ranibizumab therapy, CRT was significantly decreased after aflibercept therapy (p < 0.001), whereas there was no significant difference in BCVA (p = 0.092) between the two times. Improved BCVA was noted in 8 eyes (38.1%) after aflibercept therapy and BCVA was unchanged in 11 eyes (52.4%). Decreased CRT was noted in 18 eyes (85.7%) after aflibercept therapy.
CONCLUSIONS
Three bimonthly aflibercept injections were found to be useful in terms of improving or maintaining visual acuity, as well as reducing retinal thickness in neovascular AMD that showed limited response to 3 initial ranibizumab injections.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Up-to-date knowledge on age-related macular degeneration
Kyoung Lae Kim, Sung Pyo Park
J Korean Med Assoc. 2018;61(7):416-425. doi: 10.5124/jkma.2018.61.7.416.
Reference
-
References
1. Bressler SB, Bressler NM, Fine SL, et al. Natural course of choroi-dal neovascular membranes within the foveal avascular zone in se-nile macular degeneration. Am J Ophthalmol. 1961; 66:111–24.
Article2. Park SJ, Kwon KE, Choi NK, et al. Prevalence and incidence of exu-dative age-related macular degeneration in South Korea: a nation-wide population-based study. Ophthalmology. 2015; 122:2063–70.3. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus ver-teporfin for neovascular age-related macular degeneration. N Engl J Med. 1961; 66:111–24.
Article4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neo-vascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.
Article5. Kang S, Cho WK, Roh YJ. The efficacy of ranibizumab for choroi-dal neovascularization in age-related macular degeneration. J Korean Ophthalmol Soc. 1961; 66:111–24.
Article6. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ra-nibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 1961; 66:111–24.
Article7. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthal- mology. 1961; 66:111–24.8. Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exuda-tive age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013; 156:15–22.
Article9. Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 1961; 66:111–24.
Article10. Moon da RC, Lee DK, Kim SH, et al. Aflibercept treatment for ne-ovascular age-related macular degeneration and polypoidal choroi-dal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 1961; 66:111–24.
Article11. Kim JH, Cho NC, Kim WJ. Intravitreal aflibercept for neovascular age-related macular degeneration resistant to bevacizumab and ranibizumab. J Korean Ophthalmol Soc. 1961; 66:111–24.
Article12. Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and rani-bizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 1961; 66:111–24.
Article13. Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 1961; 66:111–24.
Article14. Niwa Y, Kakinoki M, Sawada T, et al. Ranibizumab and afli-bercept: intraocular pharmacokinetics and their effects on aqueous VEGF level in vitrectomized and nonvitrectomized macaque eyes. Invest Ophthalmol Vis Sci. 1961; 66:111–24.
Article15. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 1961; 66:111–24.
Article16. Mueller S, Agostini H, Ehlken C, et al. Patient preferences in the treatment of neovascular age-related macular degeneration: a dis-crete choice experiment. Ophthalmology. 1961; 66:111–24.17. Cruess AF, Zlateva G, Xu X, et al. Economic burden of bilateral ne-ovascular age-related macular degeneration: multi-country ob-servational study. Pharmacoeconomics. 1961; 66:111–24.18. Ruiz-Moreno JM, Coco RM, Garcia-Arumi J, et al. Burden of ill-ness of bilateral neovascular age-related macular degeneration in Spain. Curr Med Res Opin. 1961; 66:111–24.19. Oliver-Fernandez A, Bakal J, Segal S, et al. Progression of visual loss and time between initial assessment and treatment of wet age-re-lated macular degeneration. Can J Ophthalmol. 1961; 66:111–24.
Article20. Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina. 1961; 66:111–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Limited Treatment Response during Follow-up after Switching to Aflibercept in Neovascular Age-related Macular Degeneration
- Outcome of Aflibercept-Bevacizumab Alternate Dosing in Neovascular Age-related Macular Degeneration with Limited Response to Aflibercept
- Course of Neovascular Age-related Macular Degeneration that Showed Limited Response to Both Ranibizumab and Aflibercept
- Efficacy of Injection Interval Shortening in Neovascular Age-related Macular Degeneration with Limited Response to Bimonthly Aflibercept
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab