J Korean Ophthalmol Soc.
2019 Jan;60(1):40-46. 10.3341/jkos.2019.60.1.40.
Clinical Changes after Switching from Ranibizumab/Aflibercept to Bevacizumab in Exudative Age-related Macular Degeneration
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Pusan National University, Yangsan, Korea. jlee@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2431838
- DOI: http://doi.org/10.3341/jkos.2019.60.1.40
Abstract
- PURPOSE
This study was performed to investigate the changes in clinical findings after switching from ranibizumab or aflibercept to bevacizumab due to the limited number of insured injections in patients with exudative age-related macular degeneration (ARMD).
METHODS
The study population consisted of patients who had undergone intravitreal injection of ranibizumab or aflibercept for ≥ 6 months and were then treated with bevacizumab for ≥ 6 consecutive months for exudative ARMD. We evaluated best-corrected visual acuity, central subfield macular thickness, disease activity, and the number of injections for one year at the time of switching the drugs.
RESULTS
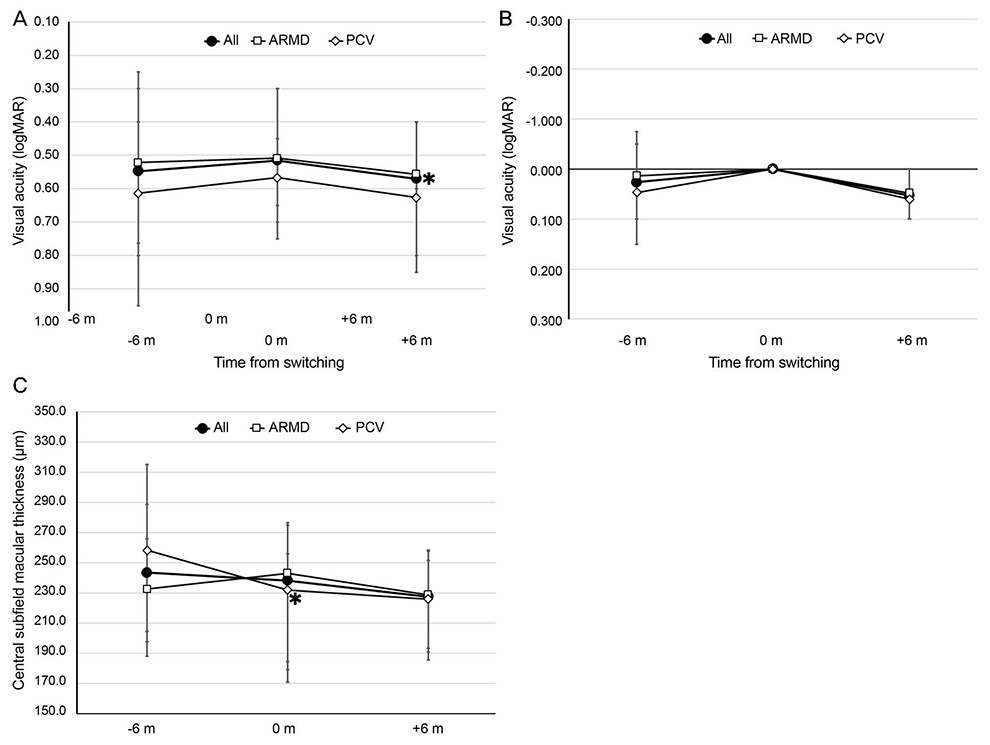
Forty patients (26 men and 14 women) were included in the analysis. The mean age was 71.9 (56-89) years old, with typical ARMD in 23 eyes, polypoidal choroidal vasculopathy in 15 eyes, and retinal angiomatous proliferation in two eyes. The number of injections for 6 months increased from 2.3 to 2.9 after switching the drugs (p < 0.001). Visual acuity was not significantly different between 6 months before and at the time of switching (LogMAR 0.55 ± 0.34 and 0.52 ± 0.27, respectively) (p = 0.300), but decreased significantly to 0.57 ± 0.34 at 6 months after switching (p = 0.018). There were no significant differences in central subfield macular thickness or disease activity.
CONCLUSIONS
Bevacizumab required more injections to achieve similar anatomical outcomes in patients with exudative ARMD treated with ranibizumab or aflibercept, and visual acuity decreased despite anatomical stability.
Keyword
MeSH Terms
Figure
Reference
-
1. Augood C, Fletcher A, Bentham G, et al. Methods for a population-based study of the prevalence of and risk factors for age-related maculopathy and macular degeneration in elderly European populations: the EUREYE study. Ophthalmic Epidemiol. 2004; 11:117–129.
Article2. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–572.
Article3. Park SJ, Lee JH, Woo SJ, et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–1765.4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.
Article5. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.
Article6. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–372.
Article7. Folk JC, Stone EM. Ranibizumab therapy for neovascular age-related macular degeneration. N Engl J Med. 2010; 363:1648–1655.
Article8. Lanzetta P, Mitchell P, Wolf S, Veritti D. Different antivascular endothelial growth factor treatments and regimens and their outcomes in neovascular age-related macular degeneration: a literature review. Br J Ophthalmol. 2013; 97:1497–1507.
Article9. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398.10. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.
Article11. Ehlken C, Jungmann S, Böhringer D, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye (Lond). 2014; 28:538–545.
Article12. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114:2179–2182.
Article13. Moisseiev E, Katz G, Moisseiev J, et al. Switching treatment for neovascular age-related macular degeneration from bevacizumab to ranibizumab: who is likely to benefit from the switch? Retina. 2015; 35:1323–1330.14. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.
Article15. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–870.
Article16. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46:726–733.
Article17. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012; 96:1–2.
Article18. Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012; 96:14–20.
Article19. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–335.
Article20. Kim JH, Cho NC, Kim WJ. Intravitreal aflibercept for neovascular age-related macular degeneration resistant to bevacizumab and ranibizumab. J Korean Ophthalmol Soc. 2015; 56:1359–1364.
Article21. Singh RP, Srivastava S, Ehlers JP, et al. A single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration previously treated with ranibizumab or bevacizumab: 6-month interim analysis. Br J Ophthalmol. 2014; 98:Suppl 1. i22–i27.22. Moon DR, Lee DK, Kim SH, et al. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 2015; 29:226–232.
Article23. Bhisitkul RB, Desai SJ, Boyer DS, et al. Fellow eye comparisons for 7-year outcomes in ranibizumab-treated AMD subjects from ANCHOR, MARINA, and HORIZON (SEVEN-UP Study). Ophthalmology. 2016; 123:1269–1277.24. Aslankurt M, Aslan L, Aksoy A, et al. The results of switching between 2 anti-VEGF drugs, bevacizumab and ranibizumab, in the treatment of neovascular age-related macular degeneration. Eur J Ophthalmol. 2013; 23:553–557.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Limited Treatment Response during Follow-up after Switching to Aflibercept in Neovascular Age-related Macular Degeneration
- Incidence of New Choroidal Neovascularization in Fellow Eyes of Patients Treated for Age-Related Macular Degeneration
- Clinical Outcomes of Aflibercept Treatment for Treatment-naive Exudative Age-related Macular Degeneration
- Course of Neovascular Age-related Macular Degeneration that Showed Limited Response to Both Ranibizumab and Aflibercept