Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Affiliations
-
- 1Department of Ophthalmology, Chonbuk National University Medical School, Jeonju, Korea. alberts123@hanmail.net
- 2Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2214550
- DOI: http://doi.org/10.3341/jkos.2015.56.9.1359
Abstract
- PURPOSE
To evaluate outcomes of intravitreal aflibercept in cases resistant to bevacizumab and ranibizumab in neovascular age-related macular degeneration.
METHODS
Twenty patients with neovascular age-related macular generation who were resistant to treatment with bevacizumab and ranibizumab were evaluated. After switching to aflibercept the best corrected visual acuity (BCVA) and central retinal thickness (CRT) were compared at baseline and at 1 month after injection. Additionally, changes in the intraretinal fluid, subretinal fluid and pigment epithelial detachment were evaluated.
RESULTS
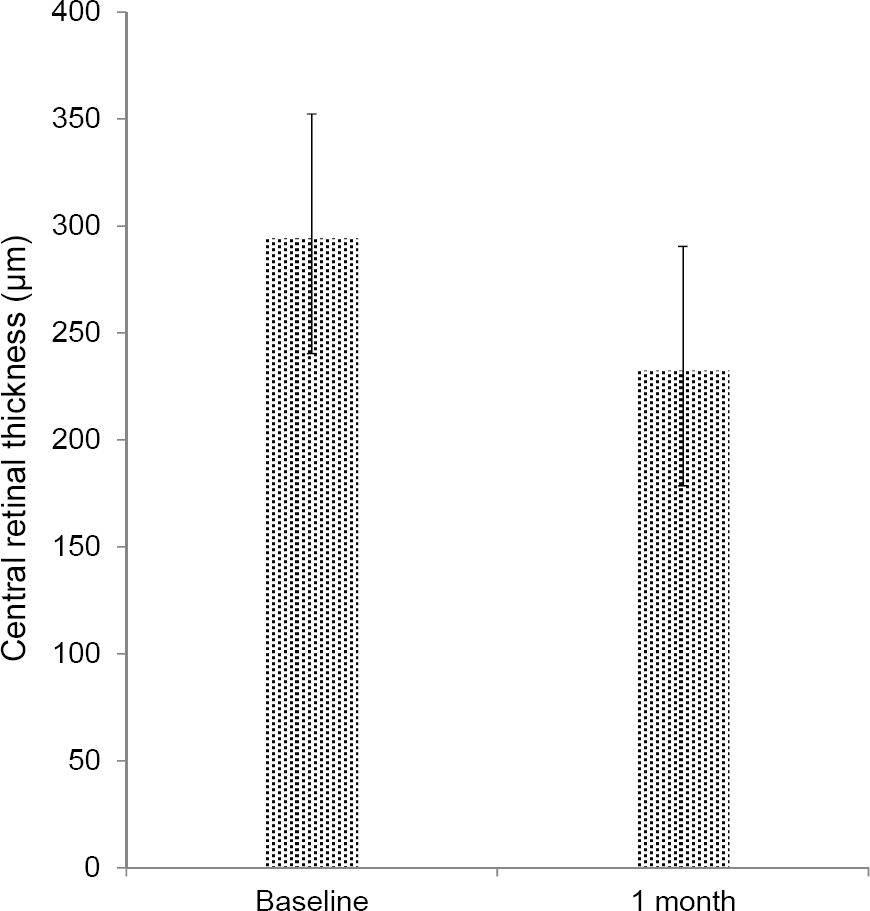
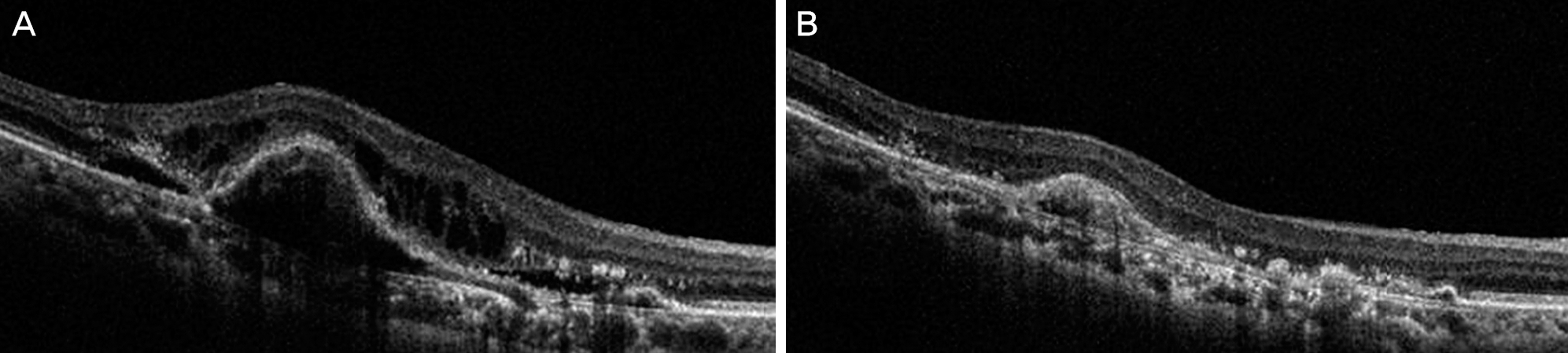
The mean BCVA was 0.83 +/- 0.56 log MAR and the mean CRT was 294.20 +/- 12.99 microm before aflibercept treatment. After switching to aflibercept the mean BCVA was 0.86 +/- 0.61 log MAR with no statistical difference (p = 0.406) and the mean CRT was decreased to 232.45 +/- 12.05 microm (p = 0.011). After 1 month of aflibercept injections, a reduction of intraretinal fluid in 4 eyes (80%), reduction of subretinal fluid in 11 eyes (78.6%) and reduction of pigment epithelial detachment in 5 eyes (50%) were observed. Increases in fluid or new lesions were not observed.
CONCLUSIONS
Aflibercept injection appears beneficial in patients with neovascular age-related macular generation who are resistant to bavacizumab and ranibizumab treatment.
MeSH Terms
Figure
Cited by 5 articles
-
Intravitreal Aflibercept Monotherapy for Treating Submacular Hemorrhage Secondary to Neovascular Age-related Macular Degeneration
Sue Hey Chae, Soh Eun Ahn, Hee Seong Yoon
J Korean Ophthalmol Soc. 2018;59(5):437-443. doi: 10.3341/jkos.2018.59.5.437.Clinical Changes after Switching from Ranibizumab/Aflibercept to Bevacizumab in Exudative Age-related Macular Degeneration
In Ho Lee, Jae Jung Lee, Han Jo Kwon, Sung Who Park, Ji Eun Lee
J Korean Ophthalmol Soc. 2019;60(1):40-46. doi: 10.3341/jkos.2019.60.1.40.Up-to-date knowledge on age-related macular degeneration
Kyoung Lae Kim, Sung Pyo Park
J Korean Med Assoc. 2018;61(7):416-425. doi: 10.5124/jkma.2018.61.7.416.Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
Kyung Min Kim, Jae Hui Kim, Young Suk Chang, Jong Woo Kim, Chul Gu Kim, Dong Won Lee
J Korean Ophthalmol Soc. 2017;58(1):62-68. doi: 10.3341/jkos.2017.58.1.62.Long-term Treatment Outcome of Intravitreal Aflibercept Monotherapy for Polypoidal Choroidal Vasculopathy
Ye Ji Kim, Sang Yun Han, Jong Woo Kim, Chul Gu Kim, Dong Won Lee, Jae Hui Kim
J Korean Ophthalmol Soc. 2018;59(3):238-245. doi: 10.3341/jkos.2018.59.3.238.
Reference
-
References
1. Friedman DS, O'Colmain BJ, Muñoz B. . Prevalence of age-re-lated macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.
Article2. Augood C, Fletcher A, Bentham G. . Methods for a pop-ulation-based study of the prevalence of and risk factors for age-re-lated maculopathy and macular degeneration in elderly European populations: the EUREYE study. Ophthalmic Epidemiol. 2004; 11:117–29.
Article3. Resnikoff S, Pascolini D, Etya'ale D. . Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82:844–51.4. Au Eong KG. Age-related macular degeneration: an emerging challenge for eye care and public health professionals in the Asia Pacific region. Ann Acad Med Singapore. 2006; 35:133–5.5. Frampton JE. Ranibizumab: a review of its use in the treatment of neovascular age-related macular degeneration. Drugs Aging. 2013; 30:331–58.
Article6. Schouten JS, La Heij EC, Webers CA. . A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1–11.
Article7. Heier JS, Brown DM, Chong V. . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.
Article8. Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW. Aflibercept for age-related macular degeneration: a game-changer or quiet ad-dition? Am J Ophthalmol. 2012; 154:222–6.9. Holash J, Davis S, Papadopoulos N. . VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002; 99:11393–8.
Article10. Papadopoulos N, Martin J, Ruan Q. . Binding and neutraliza-tion of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–85.
Article11. Dixon JA, Oliver SC, Olson JL, Mandava N. VEGF Trap-Eye for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2009; 18:1573–80.
Article12. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 2008; 92:667–8.
Article13. Chappelow AV, Kaiser PK. Neovascular age-related macular de-generation: potential therapies. Drugs. 2008; 68:1029–36.14. Rudge JS, Thurston G, Davis S. . VEGF trap as a novel anti-angiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005; 70:411–8.15. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.
Article16. Ferrara N, Damico L, Shams N. . Development of ranibizu-mab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.
Article17. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: ta-chyphylaxis or tolerance? Br J Ophthalmol. 2012; 96:1–2.18. Gasperini JL, Fawzi AA, Khondkaryan A. . Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neo- vascularisation. Br J Ophthalmol. 2012; 96:14–20.19. Cho H, Shah CP, Weber M. . Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013; 97:1032–5.
Article20. Ho VY, Yeh S, Olsen TW. . Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes pre-viously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013; 156:23–8.e2.
Article21. Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995; 36:1290–7.22. Hageman GS, Luthert PJ, Victor Chong NH. . An integrated hypothesis that considers drusen as biomarkers of immune-medi-ated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20:705–32.
Article23. Oh H, Takagi H, Takagi C. . The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999; 40:1891–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Changes after Switching from Ranibizumab/Aflibercept to Bevacizumab in Exudative Age-related Macular Degeneration
- Outcome of Aflibercept-Bevacizumab Alternate Dosing in Neovascular Age-related Macular Degeneration with Limited Response to Aflibercept
- Course of Neovascular Age-related Macular Degeneration that Showed Limited Response to Both Ranibizumab and Aflibercept
- Full-thickness Macular Hole after Intravitreal Aflibercept Injection in a Patient with Wet Age-related Macular Degeneration
- Limited Treatment Response during Follow-up after Switching to Aflibercept in Neovascular Age-related Macular Degeneration