Ann Lab Med.
2015 Jan;35(1):1-14. 10.3343/alm.2015.35.1.1.
Mitochondrial DNA Aberrations and Pathophysiological Implications in Hematopoietic Diseases, Chronic Inflammatory Diseases, and Cancers
- Affiliations
-
- 1Department of Laboratory Medicine, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Hwasun, Korea. mgshin@chonnam.ac.kr
- 2Brain Korea 21 Project, Center for Biomedical Human Resources, Chonnam National University, Gwangju, Korea.
- 3Department of Integrative Biology, University of California, Berkeley, CA, USA.
- 4Department of Cell Therapy, Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany.
- 5Environment Health Center for Childhood Leukemia and Cancer, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- KMID: 2363141
- DOI: http://doi.org/10.3343/alm.2015.35.1.1
Abstract
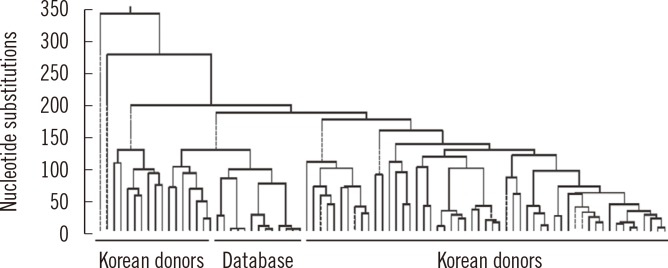
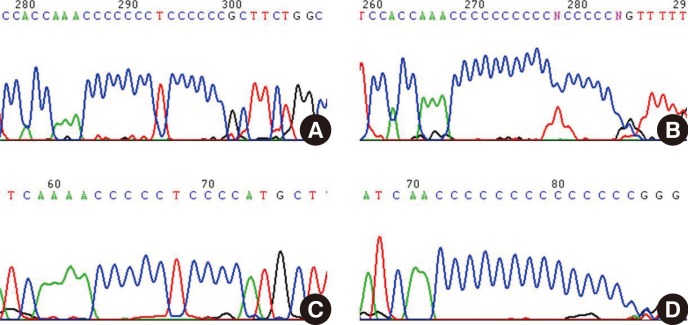
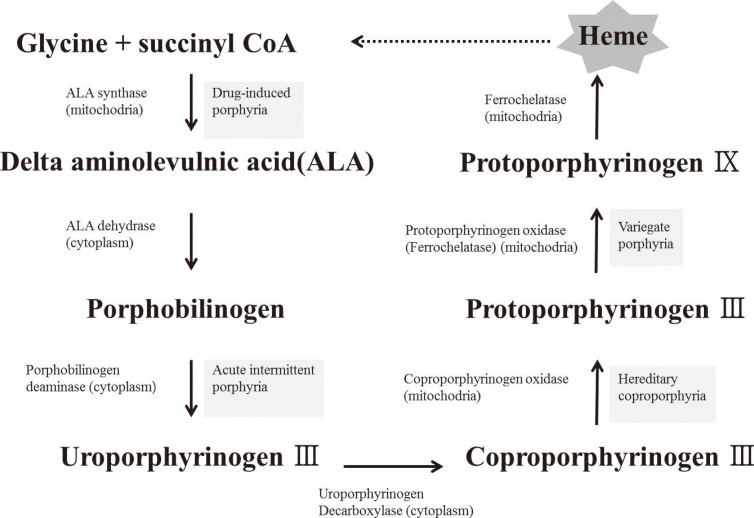
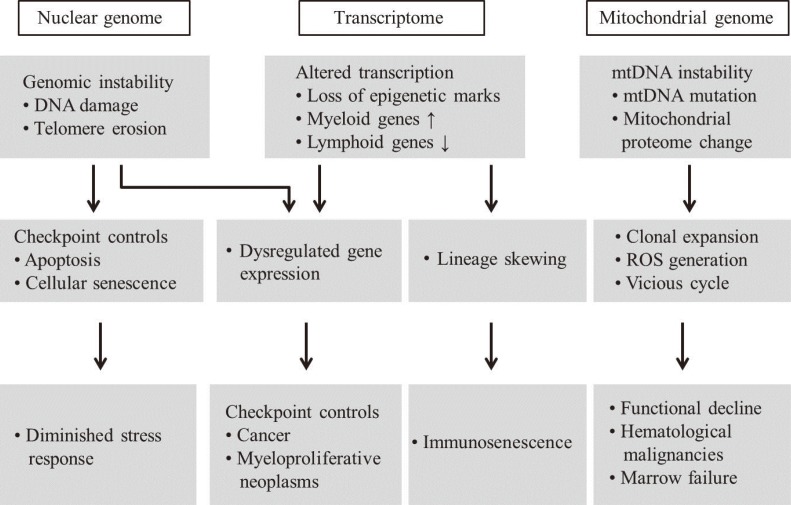
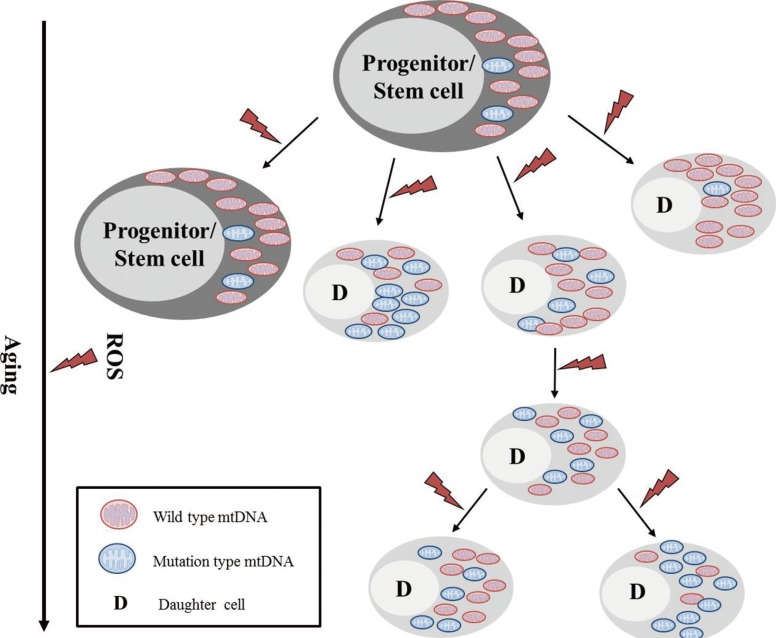
- Mitochondria are important intracellular organelles that produce energy for cellular development, differentiation, and growth. Mitochondrial DNA (mtDNA) presents a 10- to 20-fold higher susceptibility to genetic mutations owing to the lack of introns and histone proteins. The mtDNA repair system is relatively inefficient, rendering it vulnerable to reactive oxygen species (ROS) produced during ATP synthesis within the mitochondria, which can then target the mtDNA. Under conditions of chronic inflammation and excess stress, increased ROS production can overwhelm the antioxidant system, resulting in mtDNA damage. This paper reviews recent literature describing the pathophysiological implications of oxidative stress, mitochondrial dysfunction, and mitochondrial genome aberrations in aging hematopoietic stem cells, bone marrow failure syndromes, hematological malignancies, solid organ cancers, chronic inflammatory diseases, and other diseases caused by exposure to environmental hazards.
Keyword
MeSH Terms
Figure
Reference
-
1. Reeve AK, Krishnan KJ, Turnbull D. Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann N Y Acad Sci. 2008; 1147:21–29. PMID: 19076427.
Article2. Shin MG, Kajigaya S, Levin BC, Young NS. Mitochondrial DNA mutations in patients with myelodysplastic syndromes. Blood. 2003; 101:3118–3125. PMID: 12446454.
Article3. Shin MG, Kajigaya S, Tarnowka M, McCoy JP Jr, Levin BC, Young NS. Mitochondrial DNA sequence heterogeneity in circulating normal human CD34 cells and granulocytes. Blood. 2004; 103:4466–4477. PMID: 15016645.
Article4. Wallace DC. Mitochondrial DNA in aging and disease. Sci Am. 1997; 277:40–47. PMID: 9245840.
Article5. Hagberg H, Mallard C, Rousset CI, Thornton C. Mitochondria: hub of injury responses in the developing brain. Lancet Neurol. 2014; 13:217–232. PMID: 24457191.
Article6. Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet. 2001; 106:62–70. PMID: 11579426.
Article7. Berdanier CD, Everts HB. Mitochondrial DNA in aging and degenerative disease. Mutat Res. 2001; 475:169–183. PMID: 11295162.
Article8. Shin MG, Levin BC, Kim HJ, Kim HR, Lee IK, Cho D, et al. Profiling of length heteroplasmies in the human mitochondrial DNA control regions from blood cells in the Korean population. Electrophoresis. 2006; 27:1331–1340. PMID: 16502464.
Article9. Gattermann N. From sideroblastic anemia to the role of mitochondrial DNA mutations in myelodysplastic syndromes. Leuk Res. 2000; 24:141–151. PMID: 10654450.
Article10. Ergen AV, Goodell MA. Mechanisms of hematopoietic stem cell aging. Exp Gerontol. 2010; 45:286–290. PMID: 20034552.
Article11. Park CB, Larsson NG. Mitochondrial DNA mutations in disease and aging. J Cell Biol. 2011; 193:809–818. PMID: 21606204.
Article12. Shin MG, Kajigaya S, McCoy JP Jr, Levin BC, Young NS. Marked mitochondrial DNA sequence heterogeneity in single CD34+ cell clones from normal adult bone marrow. Blood. 2004; 103:553–561. PMID: 14504082.
Article13. Liu Y, Long J, Liu J. Mitochondrial free radical theory of aging: Who moved my premise? Geriatr Gerontol Int. 2014; doi: 10.1111/ggi.12296 [Epub ahead of print].
Article14. Stuart JA, Maddalena LA, Merilovich M, Robb EL. A midlife crisis for the mitochondrial free radical theory of aging. Longev Healthspan. 2014; 3:4. PMID: 24690218.
Article15. Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal. 2013; 19:1420–1445. PMID: 23642158.
Article16. Kim HR, Shin MG, Kim MJ, Kim HJ, Shin JH, Suh SP, et al. Mitochondrial DNA aberrations of bone marrow cells from patients with aplastic anemia. J Korean Med Sci. 2008; 23:1062–1067. PMID: 19119453.
Article17. He L, Luo L, Proctor SJ, Middleton PG, Blakely EL, Taylor RW, et al. Somatic mitochondrial DNA mutations in adult-onset leukaemia. Leukemia. 2003; 17:2487–2491. PMID: 14523470.
Article18. Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997; 94:514–519. PMID: 9012815.
Article19. Yao YG, Ogasawara Y, Kajigaya S, Molldrem JJ, Falcão RP, Pintão MC, et al. Mitochondrial DNA sequence variation in single cells from leukemia patients. Blood. 2007; 109:756–762. PMID: 16946307.
Article20. Kwok CS, Quah TC, Ariffin H, Tay SK, Yeoh AE. Mitochondrial D-loop polymorphisms and mitochondrial DNA content in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011; 33:e239–e244. PMID: 21646920.
Article21. Carew JS, Zhou Y, Albitar M, Carew JD, Keating MJ, Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003; 17:1437–1447. PMID: 12886229.
Article22. Cerezo M, Bandelt HJ, Martin-Guerrero I, Ardanaz M, Vega A, Carracedo A, et al. High mitochondrial DNA stability in B-cell chronic lymphocytic leukemia. PLoS One. 2009; 4:e7902. PMID: 19924307.
Article23. Lan Q, Lim U, Liu CS, Weinstein SJ, Chanock S, Bonner MR, et al. A prospective study of mitochondrial DNA copy number and risk of non-Hodgkin lymphoma. Blood. 2008; 112:4247–4249. PMID: 18711000.
Article24. Ralph SJ, Neuzil J. Mitocans, a class of emerging anti-cancer drugs. Mol Nutr Food Res. 2009; 53:7–8. PMID: 19123182.
Article25. Neuzil J, Dyason JC, Freeman R, Dong LF, Prochazka L, Wang XF, et al. Mitocans as anti-cancer agents targeting mitochondria: lessons from studies with vitamin E analogues, inhibitors of complex II. J Bioenerg Biomembr. 2007; 39:65–72. PMID: 17294131.
Article26. Shin MG, Kim HJ, Kim HR, Lee IK, Kook H, Cho D, et al. Mitochondrial DNA minisatellites as new markers for the quantitative determination of hematopoietic chimerism after allogeneic stem cell transplantation. Leukemia. 2007; 21:369–373. PMID: 17251903.
Article27. Butler JM, Levin BC. Forensic applications of mitochondrial DNA. Trends Biotechnol. 1998; 16:158–162. PMID: 9586238.
Article28. Lim SW, Kim HR, Kim HY, Huh JW, Kim YJ, Shin JH, et al. High-frequency minisatellite instability of the mitochondrial genome in colorectal cancer tissue associated with clinicopathological values. Int J Cancer. 2012; 131:1332–1341. PMID: 22120612.
Article29. Yoo JH, Suh B, Park TS, Shin MG, Choi YD, Lee CH, et al. Analysis of fluorescence in situ hybridization, mtDNA quantification, and mtDNA sequence for the detection of early bladder cancer. Cancer Genet Cytogenet. 2010; 198:107–117. PMID: 20362225.
Article30. Lee S, Han MJ, Lee KS, Back SC, Hwang D, Kim HY, et al. Frequent occurrence of mitochondrial DNA mutations in Barrett's metaplasia without the presence of dysplasia. PLoS One. 2012; 7:e37571. PMID: 22629421.
Article31. Park SY, Shin MG, Kim HR, Oh JY, Kim SH, Shin JH, et al. Alteration of mitochondrial DNA sequence and copy number in nasal polyp tissue. Mitochondrion. 2009; 9:318–325. PMID: 19426839.
Article32. Lee S, Shin MG, Jo WH, Kim MJ, Kim HR, Lee WS, et al. Association between Helicobacter pylori-related peptic ulcer tissue and somatic mitochondrial DNA mutations. Clin Chem. 2007; 53:1390–1392. PMID: 17582156.
Article33. Park HW, Ahn Y, Jeong MH, Cho JG, Park JC, Kang JC, et al. Chronic atrial fibrillation associated with somatic mitochondrial DNA mutations in human atrial tissue. J Clin Pathol. 2007; 60:948–950. PMID: 17526804.
Article34. Eom HY, Kim HR, Kim HY, Han DK, Baek HJ, Lee JH, et al. Mitochondrial DNA copy number and hnRNP A2/B1 protein: biomarkers for direct exposure of benzene. Environ Toxicol Chem. 2011; 30:2762–2770. PMID: 21919041.
Article35. Raychoudhury SS, Kubinski D. Polycyclic aromatic hydrocarbon-induced cytotoxicity in cultured rat Sertoli cells involves differential apoptotic response. Environ Health Perspect. 2003; 111:33–38. PMID: 12515676.
Article37. Castaño-Vinyals G, D'Errico A, Malats N, Kogevinas M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup Environ Med. 2004; 61:e12. PMID: 15031403.