Diabetes Metab J.
2016 Dec;40(6):473-481. 10.4093/dmj.2016.40.6.473.
Risk Factors for the Development and Progression of Diabetic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Advanced Diabetic Retinopathy
- Affiliations
-
- 1Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. kihos@catholic.ac.kr
- 2Department of Ophthalmology, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2362220
- DOI: http://doi.org/10.4093/dmj.2016.40.6.473
Abstract
- BACKGROUND
Some patients with type 2 diabetes mellitus (T2DM) do not develop diabetic kidney disease (DKD) despite the presence of advanced diabetic retinopathy (DR). We aimed to investigate the presence of DKD and its risk factors in patients with T2DM and advanced DR.
METHODS
We conducted a cross-sectional study in 317 patients with T2DM and advanced DR. The phenotypes of DKD were divided into three groups according to the urine albumin/creatinine ratio (uACR, mg/g) and estimated glomerular filtration rate (eGFR, mL/min/1.73 m²): no DKD (uACR <30 and eGFR ≥60), non-severe DKD (uACR ≥30 or eGFR <60), and severe DKD (uACR ≥30 and eGFR <60). Mean systolic and diastolic blood pressure, mean glycosylated hemoglobin (HbA1c) level, and HbA1c variability (standard deviation [SD] of serial HbA1c values or HbA1c-SD) were calculated for the preceding 2 years.
RESULTS
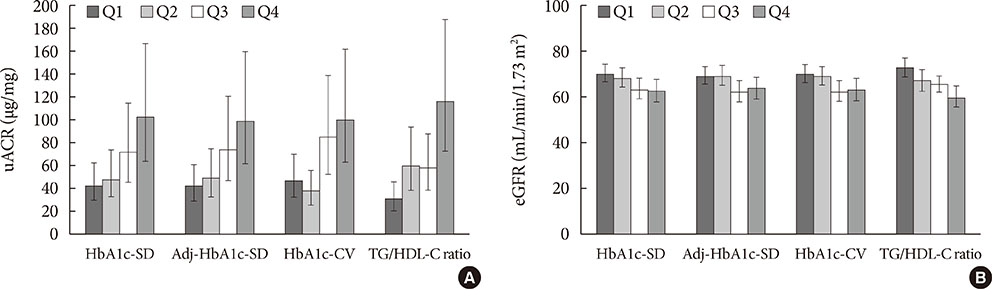
The prevalence of no DKD, non-severe DKD, and severe DKD was 37.2% (n=118), 37.0% (n=117), and 25.8% (n=82), respectively. HbA1c-SD and the triglyceride/high density lipoprotein cholesterol (TG/HDL-C) ratio correlated positively with uACR and negatively with eGFR. Multiple linear regression analyses showed that the HbA1c-SD and TG/HDL-C ratio were significantly related with eGFR. Multiple logistic regression analyses after adjusting for several risk factors showed that HbA1c-SD and the TG/HDL-C ratio were significant risk factors for severe DKD.
CONCLUSION
The prevalence of DKD was about 60% in patients with T2DM and advanced DR. HbA1c variability and TG/HDL-C ratio may affect the development and progression of DKD in these patients.
Keyword
MeSH Terms
-
Blood Pressure
Cholesterol
Cholesterol, HDL
Cross-Sectional Studies
Diabetes Mellitus, Type 2*
Diabetic Nephropathies*
Diabetic Retinopathy*
Glomerular Filtration Rate
Hemoglobin A, Glycosylated
Humans
Linear Models
Lipoproteins
Logistic Models
Phenotype
Prevalence
Risk Factors*
Triglycerides
Cholesterol
Cholesterol, HDL
Lipoproteins
Triglycerides
Figure
Cited by 2 articles
-
Albuminuria Is Associated with Steatosis Burden in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease
Eugene Han, Mi Kyung Kim, Byoung Kuk Jang, Hye Soon Kim
Diabetes Metab J. 2021;45(5):698-707. doi: 10.4093/dmj.2020.0118.Higher Prevalence and Progression Rate of Chronic Kidney Disease in Elderly Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Seok Won Park, Yong-Wook Cho, Soo-Kyung Kim
Diabetes Metab J. 2018;42(3):224-232. doi: 10.4093/dmj.2017.0065.
Reference
-
1. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. American Diabetes Association. Diabetic nephropathy. Diabetes Care. 2003; 26:Suppl 1. S94–S98.2. Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R. American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004; 27:Suppl 1. S84–S87.3. Kramer CK, Retnakaran R. Concordance of retinopathy and nephropathy over time in type 1 diabetes: an analysis of data from the Diabetes Control and Complications Trial. Diabet Med. 2013; 30:1333–1341.4. Penno G, Solini A, Zoppini G, Orsi E, Zerbini G, Trevisan R, Gruden G, Cavalot F, Laviola L, Morano S, Nicolucci A, Pugliese G. Renal Insufficiency And Cardiovascular Events (RIACE) Study Group. Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2012; 35:2317–2323.5. Magri CJ, Calleja N, Buhagiar G, Fava S, Vassallo J. Factors associated with diabetic nephropathy in subjects with proliferative retinopathy. Int Urol Nephrol. 2012; 44:197–206.6. Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Morano S, Cavalot F, Lamacchia O, Laviola L, Nicolucci A, Pugliese G. Renal Insufficiency And Cardiovascular Events Study Group. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013; 36:2301–2310.7. Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008; 31:2198–2202.8. Waden J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH. Finnish Diabetic Nephropathy Study Group. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009; 58:2649–2655.9. Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Lee YS, Shin SJ, Tai TY. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012; 55:3163–3172.10. Sugawara A, Kawai K, Motohashi S, Saito K, Kodama S, Yachi Y, Hirasawa R, Shimano H, Yamazaki K, Sone H. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012; 55:2128–2131.11. Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110:1677–1682.12. Matsumoto A, Iwashima Y, Abiko A, Morikawa A, Sekiguchi M, Eto M, Makino I. Detection of the association between a deletion polymorphism in the gene encoding angiotensin I-converting enzyme and advanced diabetic retinopathy. Diabetes Res Clin Pract. 2000; 50:195–202.13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247–254.14. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014; 37:2864–2883.15. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010; 12:288–298.16. Luk AO, Ma RC, Lau ES, Yang X, Lau WW, Yu LW, Chow FC, Chan JC, So WY. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013; 29:384–390.17. Ihnat MA, Thorpe JE, Ceriello A. Hypothesis: the 'metabolic memory', the new challenge of diabetes. Diabet Med. 2007; 24:582–586.18. Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012; 35:2076–2082.19. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–1687.20. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002; 287:2570–2581.21. Lee IT, Wang CY, Huang CN, Fu CC, Sheu WH. High triglyceride-to-HDL cholesterol ratio associated with albuminuria in type 2 diabetic subjects. J Diabetes Complications. 2013; 27:243–247.22. Chang YH, Chang DM, Lin KC, Hsieh CH, Lee YJ. High-density lipoprotein cholesterol and the risk of nephropathy in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2013; 23:751–757.23. Morton J, Zoungas S, Li Q, Patel AA, Chalmers J, Woodward M, Celermajer DS, Beulens JW, Stolk RP, Glasziou P, Ng MK. ADVANCE Collaborative Group. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care. 2012; 35:2201–2206.24. Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond). 2009; 116:87–98.25. Vaziri ND. Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J Ren Nutr. 2010; 20:5 Suppl. S35–S43.26. Attman PO. Progression of renal failure and lipids: is there evidence for a link in humans? Nephrol Dial Transplant. 1998; 13:545–547.27. Weinberg JM. Lipotoxicity. Kidney Int. 2006; 70:1560–1566.28. Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O'Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC. Fenofibrate Intervention and Event Lowering in Diabetes Study investigators. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011; 54:280–290.29. Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G. DAIS Investigators. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am J Kidney Dis. 2005; 45:485–493.30. Kim DM, Ahn CW, Park JS, Cha BS, Lim SK, Kim KR, Lee HC, Huh KB. An implication of hypertriglyceridemia in the progression of diabetic nephropathy in metabolically obese, normal weight patients with type 2 diabetes mellitus in Korea. Diabetes Res Clin Pract. 2004; 66:Suppl 1. S169–S172.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influences of Arteriosclerosis on the Development and Progression of Diabetic Retinopathy
- Clinical Analysis of Diabetic Retinopathy According to the Type of Diabetes Mellitus
- Letter: Clinical Course and Risk Factors of Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus in Korea (Diabetes Metab J 2016;40:482-93)
- Clinical Review on Diabetic Retinopathy
- Diagnosis and test for diabetic kidney disease