Korean J Ophthalmol.
2015 Feb;29(1):58-65. 10.3341/kjo.2015.29.1.58.
The Neuroprotective Effect of Maltol against Oxidative Stress on Rat Retinal Neuronal Cells
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. gjseong@yuhs.ac
- KMID: 2360139
- DOI: http://doi.org/10.3341/kjo.2015.29.1.58
Abstract
- PURPOSE
Maltol (3-hydroxy-2-methyl-4-pyrone), formed by the thermal degradation of starch, is found in coffee, caramelized foods, and Korean ginseng root. This study investigated whether maltol could rescue neuroretinal cells from oxidative injury in vitro.
METHODS
R28 cells, which are rat embryonic precursor neuroretinal cells, were exposed to hydrogen peroxide (H2O2, 0.0 to 1.5 mM) as an oxidative stress with or without maltol (0.0 to 1.0 mM). Cell viability was monitored with the lactate dehydrogenase assay and apoptosis was examined by the terminal deoxynucleotide transferase-mediated terminal uridine deoxynucleotidyl transferase nick end-labeling (TUNEL) method. To investigate the neuroprotective mechanism of maltol, the expression and phosphorylation of nuclear factor-kappa B (NF-kappaB), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 were evaluated by Western immunoblot analysis.
RESULTS
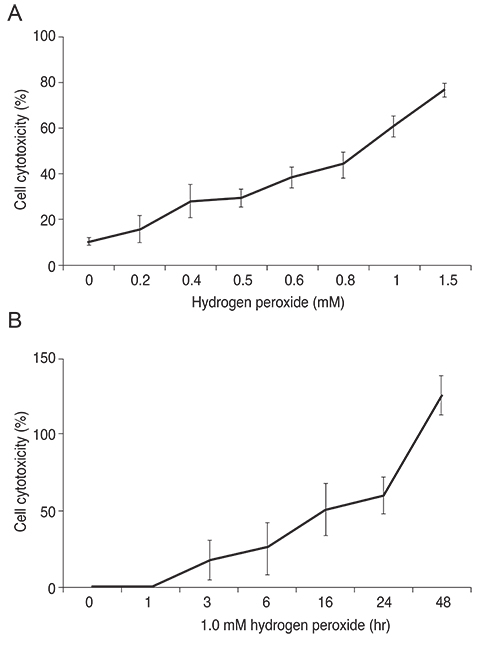
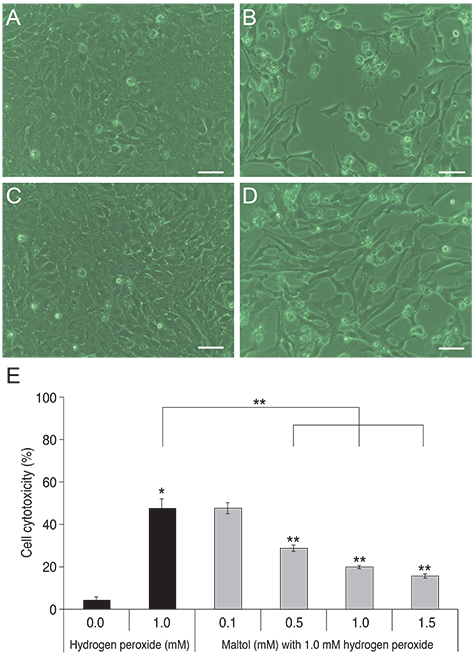
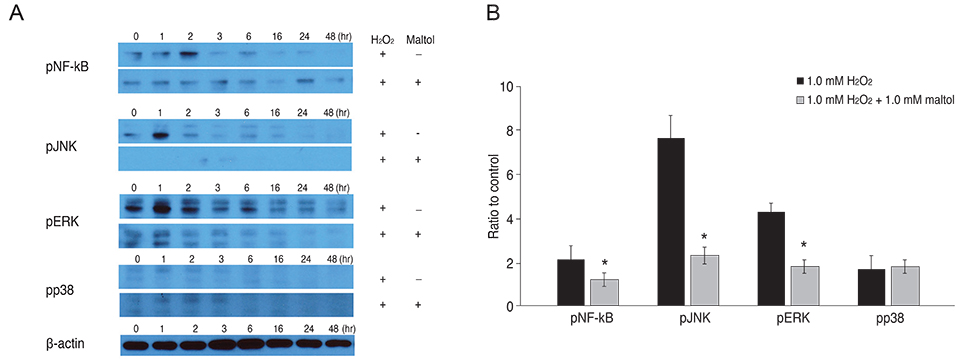
R28 cells exposed to H2O2 were found to have decreased viability in a dose- and time-dependent manner. However, H2O2-induced cytotoxicity was decreased with the addition of maltol. When R28 cells were exposed to 1.0 mM H2O2 for 24 hours, the cytotoxicity was 60.69 ± 5.71%. However, the cytotoxicity was reduced in the presence of 1.0 mM maltol. This H2O2-induced cytotoxicity caused apoptosis of R28 cells, characterized by DNA fragmentation. Apoptosis of oxidatively-stressed R28 cells with 1.0 mM H2O2 was decreased with 1.0 mM maltol, as determined by the TUNEL method. Western blot analysis showed that treatment with maltol reduced phosphorylation of NF-kappaB, ERK, and JNK, but not p38. The neuroprotective effects of maltol seemed to be related to attenuated expression of NF-kappaB, ERK, and JNK.
CONCLUSIONS
Maltol not only increased cell viability but also attenuated DNA fragmentation. The results obtained here show that maltol has neuroprotective effects against hypoxia-induced neuroretinal cell damage in R28 cells, and its effects may act through the NF-kappaB and mitogen-activated protein kinase signaling pathways.
MeSH Terms
Figure
Reference
-
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90:262–267.2. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain veterinary drug residues in food: sixty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser. 2006; (939):1–80.3. Harvey RS, Reffitt DM, Doig LA, et al. Ferric trimaltol corrects iron deficiency anaemia in patients intolerant of iron. Aliment Pharmacol Ther. 1998; 12:845–848.4. Thompson KH, Liboiron BD, Sun Y, et al. Preparation and characterization of vanadyl complexes with bidentate maltol-type ligands: in vivo comparisons of anti-diabetic therapeutic potential. J Biol Inorg Chem. 2003; 8:66–74.5. Hong YL, Pan HZ, Scott MD, Meshnick SR. Activated oxygen generation by a primaquine metabolite: inhibition by antioxidants derived from Chinese herbal remedies. Free Radic Biol Med. 1992; 12:213–218.6. Kim YB, Oh SH, Sok DE, Kim MR. Neuroprotective effect of maltol against oxidative stress in brain of mice challenged with kainic acid. Nutr Neurosci. 2004; 7:33–39.7. Yang Y, Wang J, Xu C, et al. Maltol inhibits apoptosis of human neuroblastoma cells induced by hydrogen peroxide. J Biochem Mol Biol. 2006; 39:145–149.8. Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In Vitro Cell Dev Biol Anim. 1996; 32:66–68.9. Seigel GM, Sun W, Wang J, et al. Neuronal gene expression and function in the growth-stimulated R28 retinal precursor cell line. Curr Eye Res. 2004; 28:257–269.10. Sun W, Seigel GM, Salvi RJ. Retinal precursor cells express functional ionotropic glutamate and GABA receptors. Neuroreport. 2002; 13:2421–2424.11. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998; 16:225–260.12. Van Antwerp DJ, Verma IM. Signal-induced degradation of I(kappa)B(alpha): association with NF-kappaB and the PEST sequence in I(kappa)B(alpha) are not required. Mol Cell Biol. 1996; 16:6037–6045.13. Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006; 6:111–130.14. Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006; 25:6680–6684.15. Wu T, Chiang SK, Chau FY, Tso MO. Light-induced photoreceptor degeneration may involve the NF kappa B/caspase-1 pathway in vivo. Brain Res. 2003; 967:19–26.16. Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996; 274:1383–1385.17. Nakai M, Qin ZH, Chen JF, et al. Kainic acid-induced apoptosis in rat striatum is associated with nuclear factor-kappaB activation. J Neurochem. 2000; 74:647–658.18. Fan W, Cooper NG. Glutamate-induced NFkappaB activation in the retina. Invest Ophthalmol Vis Sci. 2009; 50:917–925.19. Chen YG, Zhang C, Chiang SK, et al. Increased nuclear factor-kappa B p65 immunoreactivity following retinal ischemia and reperfusion injury in mice. J Neurosci Res. 2003; 72:125–131.20. Gupta VK, You Y, Li JC, et al. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013; 49:96–104.21. Zhou Y, Pernet V, Hauswirth WW, Di Polo A. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Mol Ther. 2005; 12:402–412.22. Pernet V, Hauswirth WW, Di Polo A. Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. J Neurochem. 2005; 93:72–83.23. Luo JM, Cen LP, Zhang XM, et al. PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci. 2007; 26:828–842.24. Cagnol S, Van Obberghen-Schilling E, Chambard JC. Prolonged activation of ERK1,2 induces FADD-independent caspase 8 activation and cell death. Apoptosis. 2006; 11:337–346.25. Stanciu M, Wang Y, Kentor R, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000; 275:12200–12206.26. Basu K, Mukhopadhyay A, Ghosh I, Datta K. Nuclear morphology and c-Jun N-terminal kinase 1 expression differentiate serum-starved oxidative stress signalling from hydrogen peroxide-induced apoptosis in retinal neuronal cell line. Cell Biol Int. 2012; 36:1021–1027.27. Ogura M, Kitamura M. Oxidant stress incites spreading of macrophages via extracellular signal-regulated kinases and p38 mitogen-activated protein kinase. J Immunol. 1998; 161:3569–3574.28. Seigel GM. R28 retinal precursor cells: the first 20 years. Mol Vis. 2014; 20:301–306.29. Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001; 86:1–12.30. Sippl C, Tamm ER. What is the nature of the RGC-5 cell line? Adv Exp Med Biol. 2014; 801:145–154.31. Krishnamoorthy RR, Clark AF, Daudt D, et al. A forensic path to RGC-5 cell line identification: lessons learned. Invest Ophthalmol Vis Sci. 2013; 54:5712–5719.32. Hironishi M, Kordek R, Yanagihara R, Garruto RM. Maltol (3-hydroxy-2-methyl-4-pyrone) toxicity in neuroblastoma cell lines and primary murine fetal hippocampal neuronal cultures. Neurodegeneration. 1996; 5:325–329.33. Gralla EJ, Stebbins RB, Coleman GL, Delahunt CS. Toxicity studies with ethyl maltol. Toxicol Appl Pharmacol. 1969; 15:604–613.34. Anwar-Mohamed A, El-Kadi AO. Induction of cytochrome P450 1a1 by the food flavoring agent, maltol. Toxicol In Vitro. 2007; 21:685–690.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Efficacy of Methanol Extract of Bidens tripartita in HT22 Cells by Neuroprotective Effect
- Protective effect of maltol on pathological response of cardiomyocyte in dystrophic mice
- Epilepsy and Oxidative Stress
- Effect of Berberine on Cell Survival in the Developing Rat Brain Damaged by MK-801
- Iron-induced cytotoxicity in cultured rat retinal neurons