Cancer Res Treat.
2016 Oct;48(4):1210-1221. 10.4143/crt.2015.374.
Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Surrogate Biomarker for Bevacizumab in Colorectal Cancer Liver Metastasis: A Single-Arm, Exploratory Trial
- Affiliations
-
- 1Department of Diagnostic Radiology, Seoul Medical Center, Seoul, Korea.
- 2Department of Diagnostic Radiology, Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. radpms@yuhs.ac
- 3Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. SSJ338@yuhs.ac
- KMID: 2356223
- DOI: http://doi.org/10.4143/crt.2015.374
Abstract
- PURPOSE
The purpose of this study is to investigate dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and plasma cytokines and angiogenic factors (CAFs) as pharmacodynamic and prognostic biomarkers of bevacizumab monotherapy in colorectal cancer with liver metastasis (CRCLM).
MATERIALS AND METHODS
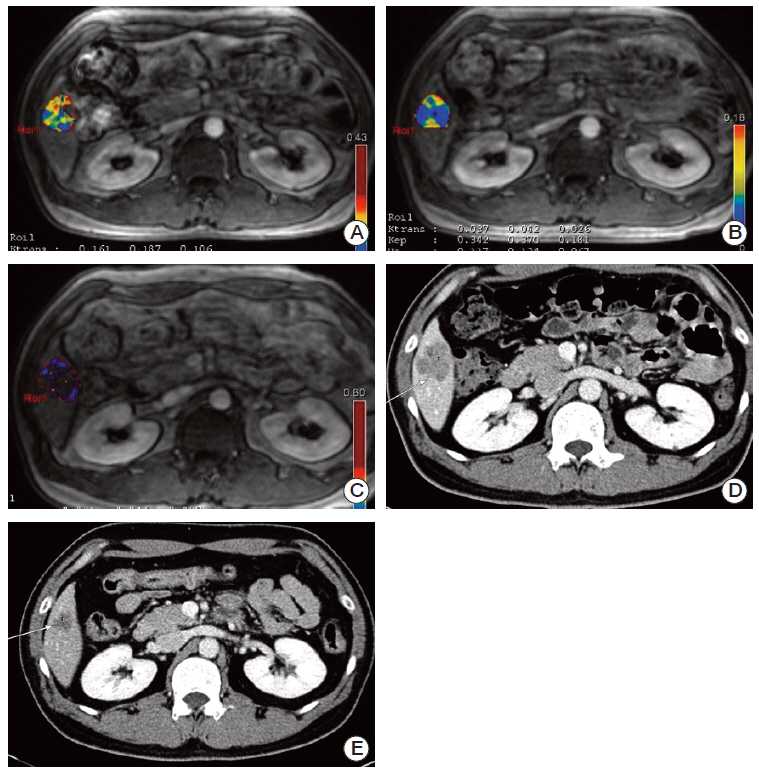
From July 2011 to March 2012, 28 patients with histologically confirmed CRCLM received bevacizumab monotherapy followed by combined FOLFOX therapy. The mean age of the patients was 57 years (range, 30 to 77 years). DCE-MRI (K(trans) and IAUC₆₀) was performed at baseline, first follow-up (3 days after bevacizumab monotherapy), and second follow-up (3 days after combined therapy). CAF levels (vascular endothelial growth factor [VEGF], placental growth factor [PlGF], and interleukin-8) were assessed on the same days. Progression-free survival (PFS) time distributions were summarized using the Kaplan-Meier method and compared using log-rank tests.
RESULTS
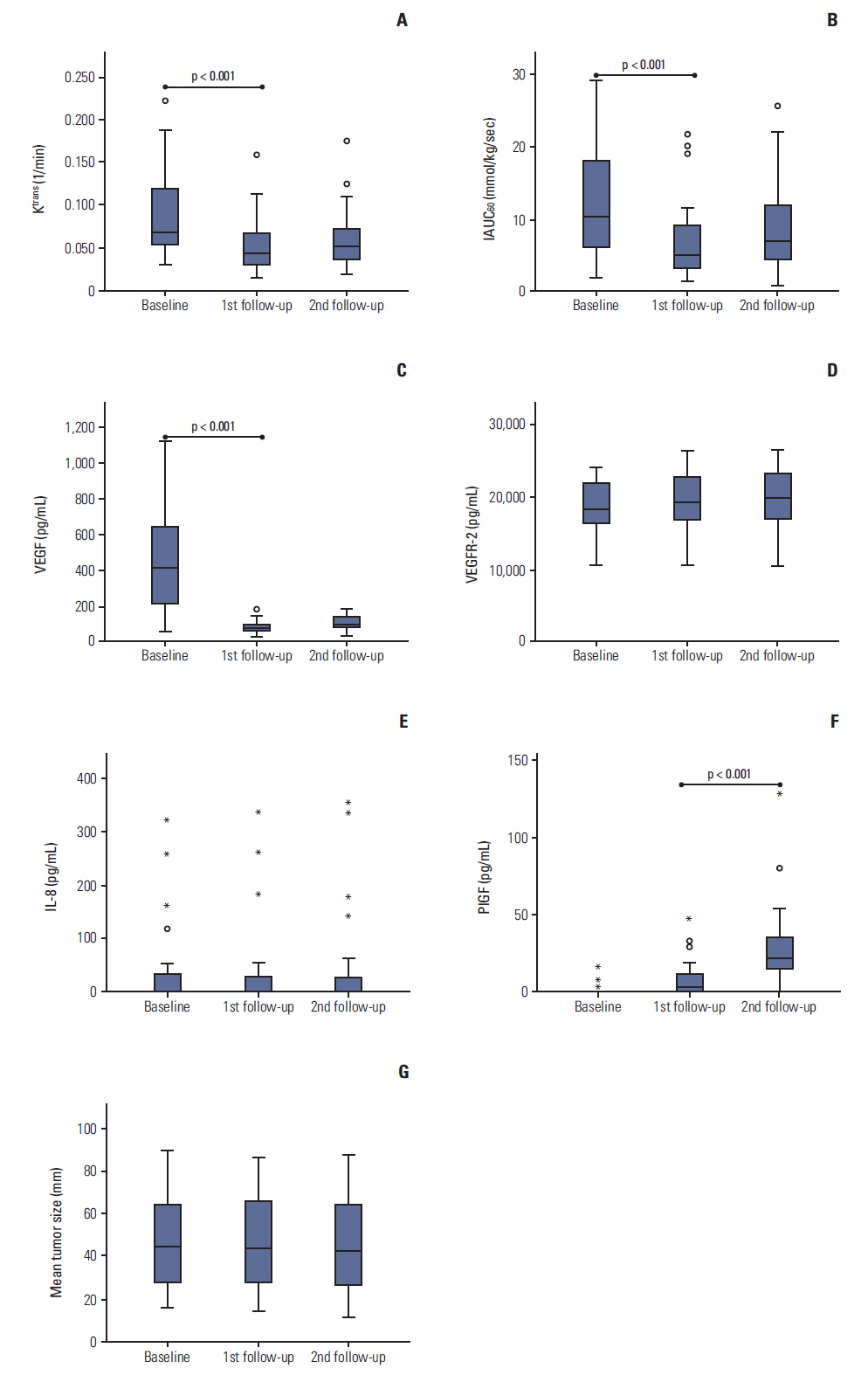
The median PFS period was 11.2 months. K(trans), IAUC₆₀, VEGF, and PlGF values on the first follow-up day were significantly different compared with baseline values. No differences were observed on the second follow-up day. A > 40% decrease in K(trans) from baseline to first follow-up was associated with a longer PFS (hazard ratio, 0.349; 95% confidence interval, 0.133 to 0.912; p=0.032). Changes in CAFs did not show correlation with PFS time.
CONCLUSION
DCE-MRI parameters and CAFs are pharmacodynamic biomarkers of bevacizumab for CRCLM. In our study, change in K(trans) at 3 days after bevacizumab monotherapy was a favorable prognostic factor; however, the value of CAFs as a prognostic biomarker was not found.
MeSH Terms
-
Angiogenesis Inducing Agents
Bevacizumab*
Biomarkers
Colorectal Neoplasms*
Cytokines
Disease-Free Survival
Endothelial Growth Factors
Follow-Up Studies
Humans
Liver*
Magnetic Resonance Imaging*
Methods
Neoplasm Metastasis*
Plasma
Vascular Endothelial Growth Factor A
Angiogenesis Inducing Agents
Bevacizumab
Biomarkers
Cytokines
Endothelial Growth Factors
Vascular Endothelial Growth Factor A
Figure
Reference
-
References
1. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatinbased chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26:2013–9.
Article2. Meyerhardt JA, Li L, Sanoff HK, Carpenter W 4th, Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012; 30:608–15.
Article3. Ducreux M, Adenis A, Pignon JP, Francois E, Chauffert B, Ichante JL, et al. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer. 2013; 49:1236–45.
Article4. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005; 307:58–62.
Article5. Hegde PS, Jubb AM, Chen D, Li NF, Meng YG, Bernaards C, et al. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res. 2013; 19:929–37.
Article6. Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009; 6:327–38.
Article7. Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010; 28:453–9.
Article8. O'Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012; 9:167–77.9. Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006; 24:769–77.
Article10. O'Connor JP, Rose CJ, Jackson A, Watson Y, Cheung S, Maders F, et al. DCE-MRI biomarkers of tumour heterogeneity predict CRC liver metastasis shrinkage following bevacizumab and FOLFOX-6. Br J Cancer. 2011; 105:139–45.11. De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J, et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer. 2012; 106:1926–33.
Article12. Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007; 25:3045–54.
Article13. Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003; 21:3955–64.
Article14. Flaherty KT, Rosen MA, Heitjan DF, Gallagher ML, Schwartz B, Schnall MD, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008; 7:496–501.
Article15. Hsu CY, Shen YC, Yu CW, Hsu C, Hu FC, Hsu CH, et al. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011; 55:858–65.
Article16. Hahn OM, Yang C, Medved M, Karczmar G, Kistner E, Karrison T, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol. 2008; 26:4572–8.
Article17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article18. Chefd'Hotel C, Hermosillo G, Faugeras O. Flows of diffeomorphisms for multimodal image registration. In : Proceedings of the 2002 IEEE International Symposium on Biomedical Imaging; 2002 Jun 7-10; Washington, DC, USA. New York: IEEE;2002. p. 753–6.19. Evelhoch JL. Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging. 1999; 10:254–9.
Article20. Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009; 27:3027–35.
Article21. Port RE, Bernstein LJ, Barboriak DP, Xu L, Roberts TP, van Bruggen N. Noncompartmental kinetic analysis of DCE-MRI data from malignant tumors: Application to glioblastoma treated with bevacizumab. Magn Reson Med. 2010; 64:408–17.
Article22. Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004; 10:145–7.
Article23. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9:669–76.
Article24. Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008; 14:6371–5.25. Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, et al. Pharmacodynamic and pharmacogenetic angiogenesis-related markers of first-line FOLFOXIRI plus bevacizumab schedule in metastatic colorectal cancer. Br J Cancer. 2011; 104:1262–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advanced Methods in Dynamic Contrast Enhanced Arterial Phase Imaging of the Liver
- Dynamic Contrast-Enhanced MR Imaging of Tietze’s Syndrome: a Case Report
- Detection of Hepatic Metastases from Colorectal Cancer: A Comparative Study between the SPIO-enhanced MR and Intraoperative Ultrasound
- Contrast-enhanced ultrasonography of the liver using SonoVue
- Multidisciplinary Functional MR Imaging for Prostate Cancer