Korean J Crit Care Med.
2016 Aug;31(3):194-201. 10.4266/kjccm.2016.00157.
Flecainide Improve Sepsis Induced Acute Lung Injury by Controlling Inflammatory Response
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea. shkwak@chonnam.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Gwangju Christian Hospital, Gwangju, Korea.
- 3Department of Anesthesiology and Pain Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- KMID: 2350903
- DOI: http://doi.org/10.4266/kjccm.2016.00157
Abstract
- BACKGROUND
Flecainide is an antiarrhythmic agent that is used primarily in the treatment of cardiac arrhythmias. Some evidences also suggest that flecainide can participate in alveolar fluid clearance and inflammatory responses. This experiment was aimed to evaluate the effects of flecainide on sepsis induced acute lung injury in a rat model.
METHODS
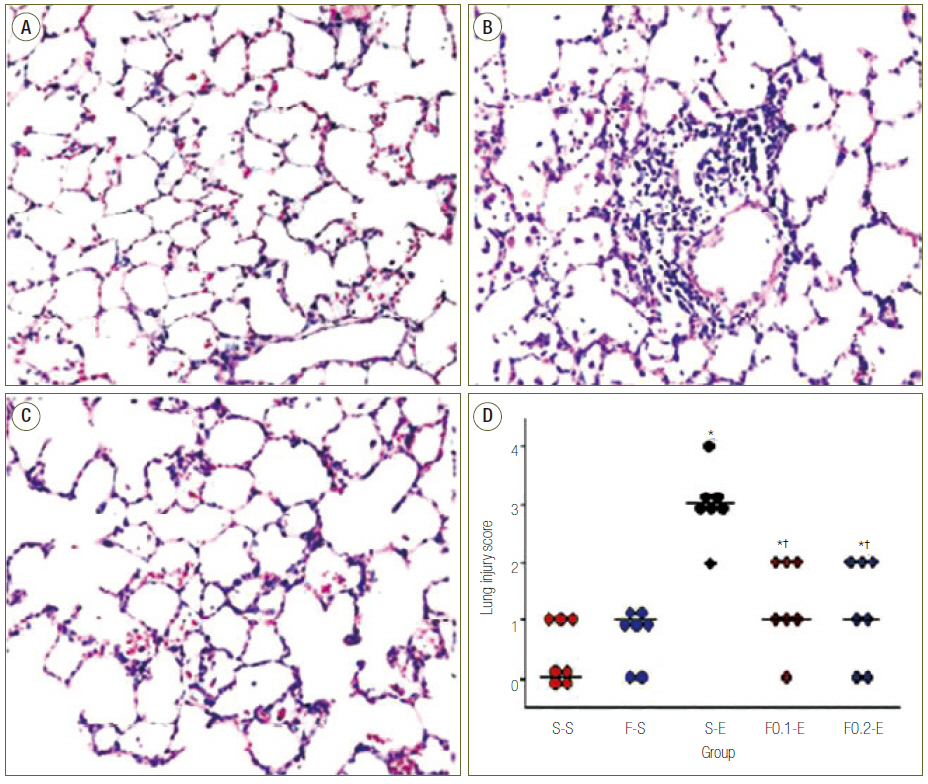
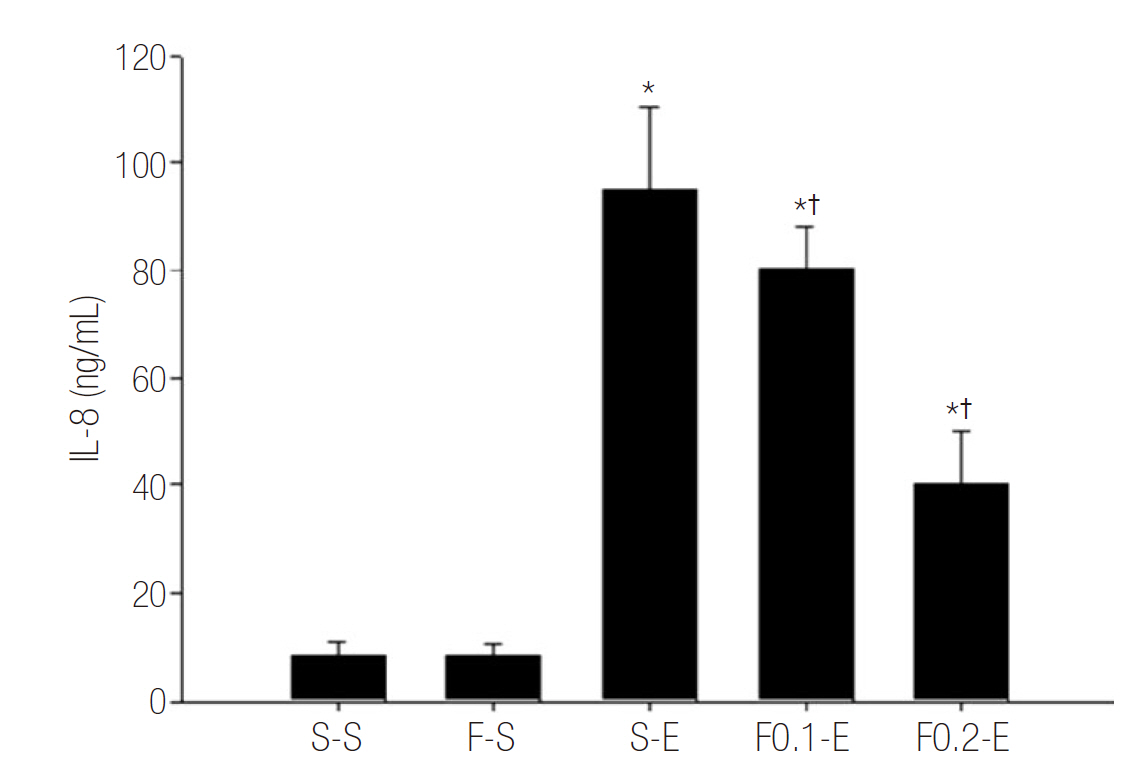
Rats were treated with subcutaneous infusion of saline or flecainide (0.1 or 0.2 mg/kg/hr) by a mini-osmotic pump. Subcutaneous infusion was started 3 hours before and continued until 8 hours after intraperitoneal injection of saline or endotoxin. Animals were sacrificed for analyses of severity of acute lung injury with wet to dry (W/D) ratio and lung injury score (LIS) in lung and inflammatory responses with level of leukocyte, polymorphonuclear neutrophils (PMNs) and inteleukin-8 (IL-8) in bronchoalveolar lavages fluid (BALF).
RESULTS
Flecainide markedly improved dose dependently sepsis induced acute lung injury as analysed by W/D ratio (from 2.24 ± 0.11 to 1.76 ± 0.09, p < 0.05) and LIS (from 3 to 1, p < 0.05), and inflammatory response as determined by leukocyte (from 443 ± 127 to 229 ± 95, p < 0.05), PMNs (from 41.43 ± 17.63 to 2.43 ± 2.61, p < 0.05) and IL-8 (from 95.00 ± 15.28 to 40.00 ± 10.21, p < 0.05) in BALF.
CONCLUSIONS
Flecanide improve sepsis induced acute lung injury in rats by controlling inflammatory responses.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Held HD, Uhlig S. Mechanisms of endotoxin-induced airway and pulmonary vascular hyperreactivity in mice. Am J Respir Crit Care Med. 2000; 162(4 Pt 1):1547–52.
Article2. Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem. 1998; 273:13469–74.
Article3. Samlowski WE, Frame RN, Logue GL. Flecainide-induced immune neutropenia. Documentation of a hapten-mediated mechanism of cell destruction. Arch Intern Med. 1987; 147:383–4.4. Kwak SH, Mitra S, Bdeir K, Strassheim D, Park JS, Kim JY, et al. The kringle domain of urokinase-type plasminogen activator potentiates LPS-induced neutrophil activation through interaction with {alpha} V{beta}3 integrins. J Leukoc Biol. 2005; 78:937–45.5. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000; 165:2950–4.6. Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol. 2002; 169:6401–7.
Article7. Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli GF. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther. 2000; 13:61–9.
Article8. Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997; 202:49–57.
Article9. Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995; 332:27–37.
Article10. Saito T, Yamamoto T, Kazawa T, Gejyo H, Naito M. Expression of toll-like receptor 2 and 4 in lipopolysaccharide-induced lung injury in mouse. Cell Tissue Res. 2005; 321:75–88.
Article11. Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004; 279:2712–8.12. Roos-Engstrand E, Wallin A, Bucht A, Pourazar J, Sandström T, Blomberg A. Increased expression of p38 MAPK in human bronchial epithelium after lipopolysaccharide exposure. Eur Respir J. 2005; 25:797–803.
Article13. Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, et al. Interleukin-8 and development of adult respiratory distress syndrome in atrisk patient groups. Lancet. 1993; 341:643–7.
Article14. Reinhart K, Meisner M, Brunkhorst FM. Markers for sepsis diagnosis: what is useful? Crit Care Clin. 2006; 22:503–19. ix-x.
Article15. O’Brodovich HM. The role of active Na+ transport by lung epithelium in the clearance of airspace fluid. New Horiz. 1995; 3:240–7.16. Baines DL, Albert AP, Hazell MJ, Gambling L, Woollhead AM, Dockrell ME. Lipopolysaccharide modifies amiloride-sensitive Na+ transport processes across human airway cells: role of mitogen-activated protein kinases ERK 1/2 and 5. Pflugers Arch. 2010; 459:451–63.
Article17. Wyncoll DL, Evans TW. Acute respiratory distress syndrome. Lancet. 1999; 354:497–501.
Article18. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001; 163:1376–83.
Article19. Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med. 2001; 163:1293–4.
Article20. Matthay MA, Clerici C, Saumon G. Invited review: Active fluid clearance from the distal air spaces of the lung. J Appl Physiol. 2002; 93:1533–41.21. Egli M, Duplain H, Lepori M, Cook S, Nicod P, Hummler E, et al. Defective respiratory amiloride-sensitive sodium transport predisposes to pulmonary oedema and delays its resolution in mice. J Physiol. 2004; 560(Pt 3):857–65.
Article22. Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L1137–45.
Article23. Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, et al. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol. 2001; 167:6601–8.
Article24. Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol. 2006; 534:1–11.
Article25. Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens. 2008; 17:526–32.
Article26. Dagenais A, Fréchette R, Yamagata Y, Yamagata T, Carmel JF, Clermont ME, et al. Downregulation of ENaC activity and expression by TNF-alpha in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004; 286:L301–11.27. Ye X, Acharya R, Herbert JB, Hamilton SE, Folkesson HG. IL-1beta stimulates alveolar fluid absorption in fetal guinea pig lungs via the hypothalamuspituitary-adrenal gland axis. Am J Physiol Lung Cell Mol Physiol. 2004; 286:L756–66.28. de Seigneux S, Leroy V, Ghzili H, Rousselot M, Nielsen S, Rossier BC, et al. NF-kappaB inhibits sodium transport via down-regulation of SGK1 in renal collecting duct principal cells. J Biol Chem. 2008; 283:25671–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Flecainide Acetate on Inflammatory-Immune Response in Lipopolysaccharide-Stimulated Neutrophils and on Mortality in Septic Rats
- Ventilation accelerates lung injury in septic mice
- The effects of BMS-470539 on lipopolysaccharide-induced acute lung injury
- Sepsis and Acute Respiratory Distress Syndrome: Recent Update
- Flecainide-Induced Torsade de Pointes Successfully Treated with Intensive Pharmacological Therapy