J Bone Metab.
2016 Aug;23(3):165-173. 10.11005/jbm.2016.23.3.165.
Pamidronate Down-regulates Tumor Necrosis Factor-alpha Induced Matrix Metalloproteinases Expression in Human Intervertebral Disc Cells
- Affiliations
-
- 1The Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. shmoon@yuhs.ac
- 2Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Orthopedic Surgery, Catholic Kwandong University College of Medicine, Incheon, Korea.
- KMID: 2350813
- DOI: http://doi.org/10.11005/jbm.2016.23.3.165
Abstract
- BACKGROUND
N-containing bisphosphonates (BPs), such as pamidronate and risedronate, can inhibit osteoclastic function and reduce osteoclast number by inducing apoptotic cell death in osteoclasts. The aim of this study is to demonstrate the effect of pamidronate, second generation nitrogen-containing BPs and to elucidate matrix metallo-proteinases (MMPs) mRNA expression under serum starvation and/or tumor necrosis factor alpha (TNF-α) stimulation on metabolism of intervertebral disc (IVD) cells in vitro.
METHODS
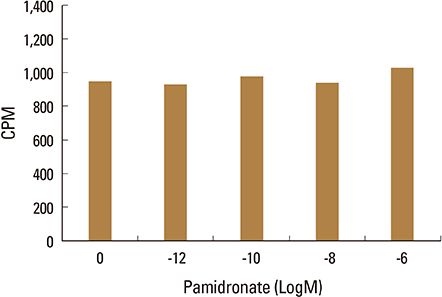
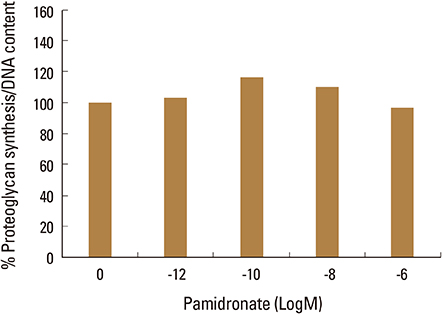
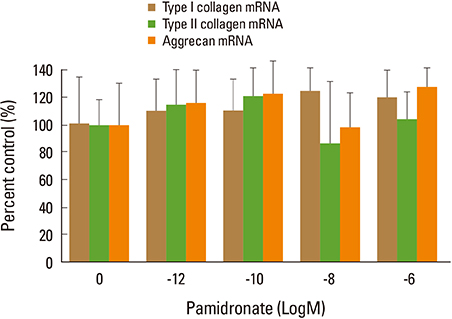
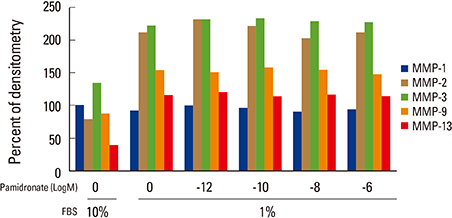
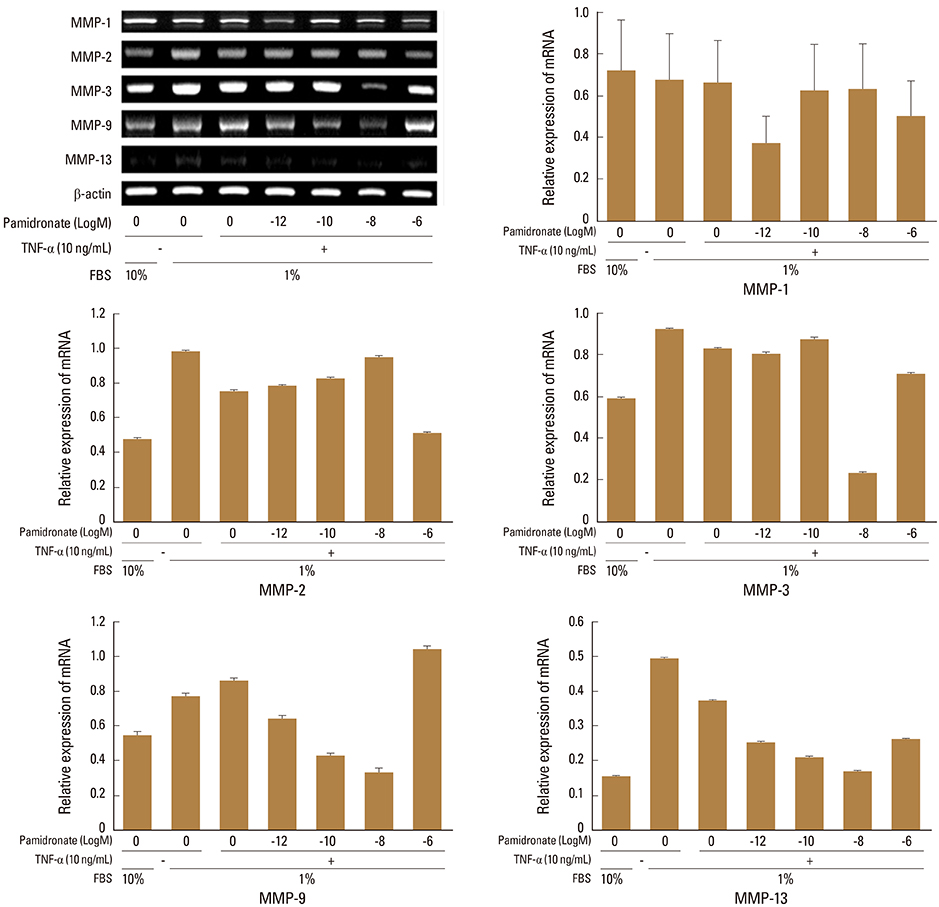
Firstly, to test the effect of pamidronate on IVD cells in vitro, various concentrations (10⻹², 10⻹â°, 10â»â¸, and 10â»â¶ M) of pamidronate were administered to IVD cells. Then DNA and proteoglycan synthesis were measured and messenger RNA (mRNA) expressions of type I collagen, type II collagen, and aggrecan were analyzed. Secondly, to elucidate the expression of MMPs mRNA in human IVD cells under the lower serum status, IVD cells were cultivated in full serum or 1% serum. Thirdly, to elucidate the expression of MMPs mRNA in IVD cells under the stimulation of 1% serum and TNF-α (10 ng/mL) In this study, IVD cells were cultivated in three dimensional alginate bead.
RESULTS
Under the lower serum culture, IVD cells in alginate beads showed upregulation of MMP 2, 3, 9, 13 mRNA. The cells in lower serum and TNF-α also demonstrated upregulation of MMP-2, 3, 9, and 13 mRNA. The cells with various doses of pamidronate and lower serum and TNF-α were reveled partial down-regulation of MMPs.
CONCLUSIONS
Pamidronate, N-containing second generation BPs, was safe in metabolism of IVD in vitro maintaining chondrogenic phenotype and matrix synthesis, and down-regulated TNF-α induced MMPs expression.
Keyword
MeSH Terms
-
Aggrecans
Cell Death
Collagen
Collagen Type I
Collagen Type II
Diphosphonates
DNA
Down-Regulation
Humans*
In Vitro Techniques
Intervertebral Disc*
Matrix Metalloproteinases*
Metabolism
Osteoclasts
Phenotype
Proteoglycans
Risedronate Sodium
RNA, Messenger
Starvation
Tumor Necrosis Factor-alpha*
Up-Regulation
Aggrecans
Collagen
Collagen Type I
Collagen Type II
DNA
Diphosphonates
Matrix Metalloproteinases
Proteoglycans
RNA, Messenger
Risedronate Sodium
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995; 10:1478–1487.
Article2. Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999; 25:97–106.
Article3. Suri S, Mönkkönen J, Taskinen M, et al. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone. 2001; 29:336–343.
Article4. Alakangas A, Selander K, Mulari M, et al. Alendronate disturbs vesicular trafficking in osteoclasts. Calcif Tissue Int. 2002; 70:40–47.
Article5. Mayahara M, Sasaki T. Cellular mechanism of inhibition of osteoclastic resorption of bone and calcified cartilage by long-term pamidronate administration in ovariectomized mature rats. Anat Rec A Discov Mol Cell Evol Biol. 2003; 274:817–826.
Article6. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002; 112:281–289.
Article7. Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002; 84-a:236–249.
Article8. Green JR. Antitumor effects of bisphosphonates. Cancer. 2003; 97:840–847.
Article9. Van Offel JF, Schuerwegh AJ, Bridts CH, et al. Effect of bisphosphonates on viability, proliferation, and dexamethasone-induced apoptosis of articular chondrocytes. Ann Rheum Dis. 2002; 61:925–928.
Article10. Garnero P, Christgau S, Delmas PD. The bisphosphonate zoledronate decreases type II collagen breakdown in patients with Paget's disease of bone. Bone. 2001; 28:461–464.
Article11. Podworny NV, Kandel RA, Renlund RC, et al. Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol. 1999; 26:1972–1982.12. Hardingham TE, Adams P. A method for the determination of hyaluronate in the presence of other glycosaminoglycans and its application to human intervertebral disc. Biochem J. 1976; 159:143–147.
Article13. Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987; 5:198–205.
Article14. Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992; 10:665–676.
Article15. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995; 20:1307–1314.
Article16. Butler D, Trafimow JH, Andersson GB, et al. Discs degenerate before facets. Spine (Phila Pa 1976). 1990; 15:111–113.
Article17. Hiyama A, Sakai D, Risbud MV, et al. Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010; 62:3036–3047.
Article18. Erwin WM, Islam D, Inman RD, et al. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011; 13:R215.
Article19. Wang M, Tang D, Shu B, et al. Conditional activation of beta-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012; 64:2611–2623.
Article20. Sato S, Kimura A, Ozdemir J, et al. The distinct role of the Runx proteins in chondrocyte differentiation and intervertebral disc degeneration: findings in murine models and in human disease. Arthritis Rheum. 2008; 58:2764–2775.
Article21. Wang H, Liu H, Zheng ZM, et al. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis. 2011; 16:990–1003.
Article22. Pratsinis H, Constantinou V, Pavlakis K, et al. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012; 30:958–964.
Article23. Studer RK, Vo N, Sowa G, et al. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-alpha. Spine (Phila Pa 1976). 2011; 36:593–599.24. Moon HJ, Kim JH, Lee HS, et al. Annulus fibrosus cells interact with neuron-like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine (Phila Pa 1976). 2012; 37:2–9.
Article25. Melrose J, Shu C, Young C, et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa 1976). 2012; 37:18–25.
Article26. Yurube T, Takada T, Suzuki T, et al. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012; 14:R51.
Article27. Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012; 1824:133–145.
Article28. Kim MS, Kim JH, Lee MR, et al. Effects of alendronate on a disintegrin and metalloproteinase with thrombospondin motifs expression in the developing epiphyseal cartilage in rats. Anat Histol Embryol. 2009; 38:154–160.
Article29. Teronen O, Heikkilä P, Konttinen YT, et al. MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci. 1999; 878:453–465.
Article30. Bone HG, Hosking D, Devogelaer JP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004; 350:1189–1199.
Article31. Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995; 186(Pt 1):43–53.32. Maldonado BA, Oegema TR Jr. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992; 10:677–690.
Article33. Verbruggen A, De Clerck LS, Bridts CH, et al. Influence of blood and synovial fluid immune complexes of patients with rheumatoid arthritis on production of nitric oxide and growth and viability of chondrocytes. J Rheumatol. 2000; 27:35–40.34. Igarashi H, Kouro T, Yokota T, et al. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci U S A. 2001; 98:15131–15136.
Article35. Lemon WJ, Bernert H, Sun H, et al. Identification of candidate lung cancer susceptibility genes in mouse using oligonucleotide arrays. J Med Genet. 2002; 39:644–655.
Article36. Brennan FM, Smith NM, Owen S, et al. Resting CD4+ effector memory T cells are precursors of bystander-activated effectors: a surrogate model of rheumatoid arthritis synovial T-cell function. Arthritis Res Ther. 2008; 10:R36.
Article37. Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008; 118:3537–3545.
Article38. Williams LM, Lali F, Willetts K, et al. Rac mediates TNF-induced cytokine production via modulation of NF-kappaB. Mol Immunol. 2008; 45:2446–2454.39. Reinholz GG, Getz B, Pederson L, et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000; 60:6001–6007.40. Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999; 104:1363–1374.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Human Recombinant Interferon-Gamma and Tumor Necrosis Factor-Alpha on Expressions of Matrix Metalloproteinases and Their Activity Human Bladder Cancer Cell Lines
- Role of Tumor Necrosis Factor Alpha, Interleukin 8 and Dexamethasone in the FAK Expression by Human Nucleus Pulposus Cells
- Macrophage Infiltrations and Expressions of Matrix Metallproteinases in Intervertebral Disc
- Synthetic Prion Peptide 106-126 Resulted in an Increase Matrix Metalloproteinases and Inflammatory Cytokines from Rat Astrocytes and Microglial Cells
- Clinical and Immunohistochemical Characteristics of Mucoceles