J Korean Ophthalmol Soc.
2012 Jun;53(6):849-855.
Effects of Simvastatin on the Expression of VEGF in Human Retinal Pigment Epithelial Cells
- Affiliations
-
- 1Department of Ophthalmology and Inha Vision Science Laboratory, Inha University School of Medicine, Incheon, Korea. hschin@inha.ac.kr
Abstract
- PURPOSE
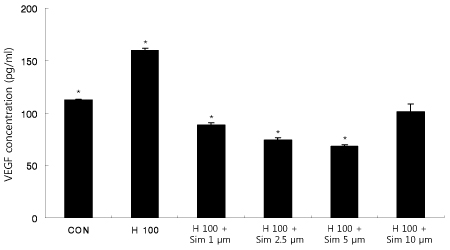
To examine the effect of simvastatin on vascular endothelial growth factor (VEGF) expression in cultured human retinal pigment epithelial (RPE) cells under oxidative stress.
METHODS
RPE cell viability was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay after 24 hours of incubation with various concentrations of simvastatin or H2O2. Cultured human RPE cells were pretreated with various concentrations of simvastatin and then incubated with 100 microm H2O2. After 24 hours of incubation, an enzyme-linked immunosorbent assay (ELISA) was performed to evaluate the expression of VEGF.
RESULTS
Simvastatin showed no toxicity up to 10 microm, but cell viability gradually decreased with increased concentration of simvastatin. Human RPE cells showed increased VEGF expression when exposed only to H2O2. When RPE cells were preincubated with simvastatin and later exposed to H2O2, VEGF expression was relatively lower.
CONCLUSIONS
Simvastatin downregulated the expression of VEGF in human RPE cells under oxidative stress. Simvastatin may have some clinical benefits in preventing retinal diseases associated with VEGF.
Keyword
MeSH Terms
Figure
Reference
-
1. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992. 99:933–943.2. Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991. 98:1128–1134.3. Ferris FL 3rd. Senile macular degeneration: review of epidemiologic features. Am J Epidemiol. 1983. 118:132–151.4. Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984. 102:1640–1642.5. Cho SW, Bae JH, Song SJ. Anatomical non-responder to intravitreal bevacizumab for neovascular age-related macular degeneration. J Korean Ophthalmol Soc. 2010. 51:1464–1470.6. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996. 86:353–364.7. Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005. 40:352–368.8. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007. 144:850–857.9. Gragoudas ES, Adamis AP, Cunningham ET Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004. 351:2805–2816.10. Cleary CA, Jungkim S, Ravikumar K, et al. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6- and 9- month results. Eye (Lond). 2008. 22:82–86.11. Kim YD, Park YC, Choi GJ. Expression of angiogeneis-related factors in retinal pigment epithelial cells under hypoxia. J Korean Ophthalmol Soc. 2006. 47:629–636.12. Kim JM, Kim JY, Lee YH, Choi GJ. Angiogenesis according to expressive change of angiogenic related factor in human RPE under oxidative stress. J Korean Ophthalmol Soc. 2005. 46:366–376.13. Boyd SR, Zachary I, Chakravarthy U, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol. 2002. 120:1644–1650.14. Ogata N, Nishikawa M, Nishimura T, et al. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002. 134:348–353.15. Lee YC, Yoon TJ, Choi GJ, Kim DH. Effect of triamcinolone on angiogenesis-related factors of cultured retinal pigment epithelial cells. J Korean Ophthalmol Soc. 2009. 50:594–602.16. Bartoli M, Al-Shabrawey M, Labazi M, et al. HMG-CoA reductase inhibitors (statin) prevents retinal neovascularization in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2009. 50:4934–4940.17. García Layana A, Salinas Alamán A, Recalde Maestre S, Fernández Robredo P. [Antioxidants and ARMD]. Arch Soc Esp Oftalmol. 2007. 82:397–398.18. Guymer RH, Dimitrov PN, Varsamidis M, et al. Can HMG Co-A reductase inhibitors ("statins") slow the progression of age-related macular degeneration? The age-related maculopathy statin study (ARMSS). Clin Interv Aging. 2008. 3:581–593.19. Tan JS, Mitchell P, Rochtchina E, Wang JJ. Statins and the long-term risk of incident age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007. 143:685–687.20. Peponis V, Chalkiadakis SE, Bonovas S, Sitaras NM. The controversy over the association between statins use and progression of age-related macular degeneration: a mini review. Clin Ophthalmol. 2010. 4:865–869.21. Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 2010. 12:e34.22. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999. 285:245–248.23. Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997. 22:251–256.24. Raymond L, Jacobson B. Isolation and identification of stimulatory and inhibitory cell growth factors in bovine vitreous. Exp Eye Res. 1982. 34:267–286.25. Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004. 242:91–101.26. Ohno-Matsui K, Morita I, Tombran-Tink J, et al. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol. 2001. 189:323–333.27. Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol. 1995. 23:293–298.28. Wu T, Fujihara M, Tian J, et al. Apolipoprotein B100 secretion by cultured ARPE-19 cells is modulated by alteration of cholesterol levels. J Neurochem. 2010. 114:1734–1744.29. Jaumdally RJ, Goon PK, Varma C, et al. Effects of atorvastatin on circulating CD34+/CD133+/ CD45- progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med. 2010. 267:385–393.30. Vincent L, Soria C, Mirshahi F, et al. Cerivastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme a reductase, inhibits endothelial cell proliferation induced by angiogenic factors in vitro and angiogenesis in in vivo models. Arterioscler Thromb Vasc Biol. 2002. 22:623–629.31. Park HJ, Zhang Y, Georgescu SP, et al. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006. 2:93–102.32. Hata Y, Miura M, Asato R, et al. Antiangiogenic mechanisms of simvastatin in retinal endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2010. 248:667–673.33. Bartoli M, Al-Shabrawey M, Labazi M, et al. HMG-CoA reductase inhibitors (statin) prevents retinal neovascularization in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2009. 50:4934–4940.34. Lutty G, Grunwald J, Majji AB, et al. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999. 5:35.35. Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010. 112:1584–1592.36. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004. 25:581–611.37. Shalev V, Sror M, Goldshtein I, et al. Statin use and the risk of age related macular degeneration in a large health organization in Israel. Ophthalmic Epidemiol. 2011. 18:83–90.38. Fong DS, Contreras R. Recent statin use and 1-year incidence of exudative age-related macular degeneration. Am J Ophthalmol. 2010. 149:955–958.39. Maguire MG, Ying GS, McCannel CA, et al. Statin use and the incidence of advanced age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2009. 116:2381–2385.40. Kaiserman N, Vinker S, Kaiserman I. Statins do not decrease the risk for wet age-related macular degeneration. Curr Eye Res. 2009. 34:304–310.41. Chuo JY, Wiens M, Etminan M, Maberley DA. Use of lipid-lowering agents for the prevention of age-related macular degeneration: a meta-analysis of observational studies. Ophthalmic Epidemiol. 2007. 14:367–374.42. Sagara N, Kawaji T, Takano A, et al. Effect of pitavastatin on experimental choroidal neovascularization in rats. Exp Eye Res. 2007. 84:1074–1080.43. Zambarakji HJ, Nakazawa T, Connolly E, et al. Dose-dependent effect of pitavastatin on VEGF and angiogenesis in a mouse model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006. 47:2623–2631.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Simvastatin on the Expression of Catalase in Human Retinal Pigment Epithelial Cells
- Effect of Triamcinolone on Angiogenesis-related Factors of Cultured Retinal Pigment Epithelial Cells
- Suppression of VEGF by Aminoguanidine in RPE Cells Cultured in the Hyperglycemic Condition
- Growth Patterns of Human Retinal Pigment Epithelium in Vitro
- The Effect of 5-Fluorouraci1 on the Activity of the Retinal Pigment Epithelium in Vitro