J Nutr Health.
2015 Feb;48(1):1-8. 10.4163/jnh.2015.48.1.1.
Effects of an aqueous extract of purple sweet potato on nonalcoholic fatty liver in high fat/cholesterol-fed mice
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 120-750, Korea. orank@ewha.ac.kr
- 2Department of Nutritional Science and Food Management, Soongeui Women's College, Seoul 100-751, Korea.
- KMID: 2327171
- DOI: http://doi.org/10.4163/jnh.2015.48.1.1
Abstract
- PURPOSE
Anthocyanins from purple sweet potato (PSP) have been investigated in vitro and in animals and found to have a protective effect against oxidative hepatic damage. In this study, we investigated that aqueous extract of PSP can ameliorate the dysfunction of lipid metabolism in mice fed a high fat/cholesterol diet.
METHODS
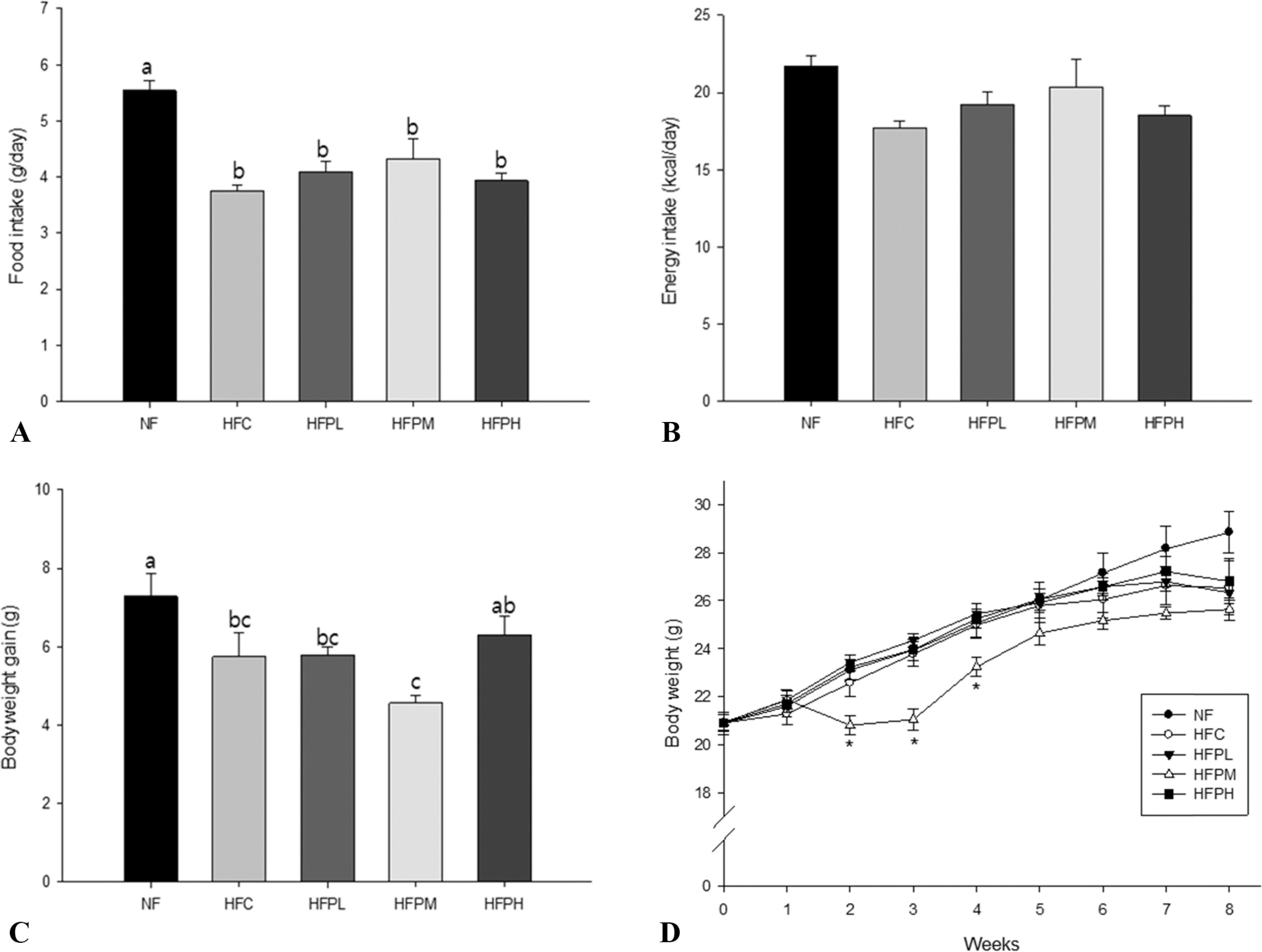
Forty C57BL/6J mice were randomly divided into 5 groups (n = 8) and fed one of the following diets for 8 weeks; normal fat (NF) diet; high fat/cholesterol (HFC) diet; HFC with 1.25% PSP (HFPL) diet; HFC with 2.5% PSP (HFPM) diet; HFC with 5% PSP (HFPH) diet.
RESULTS
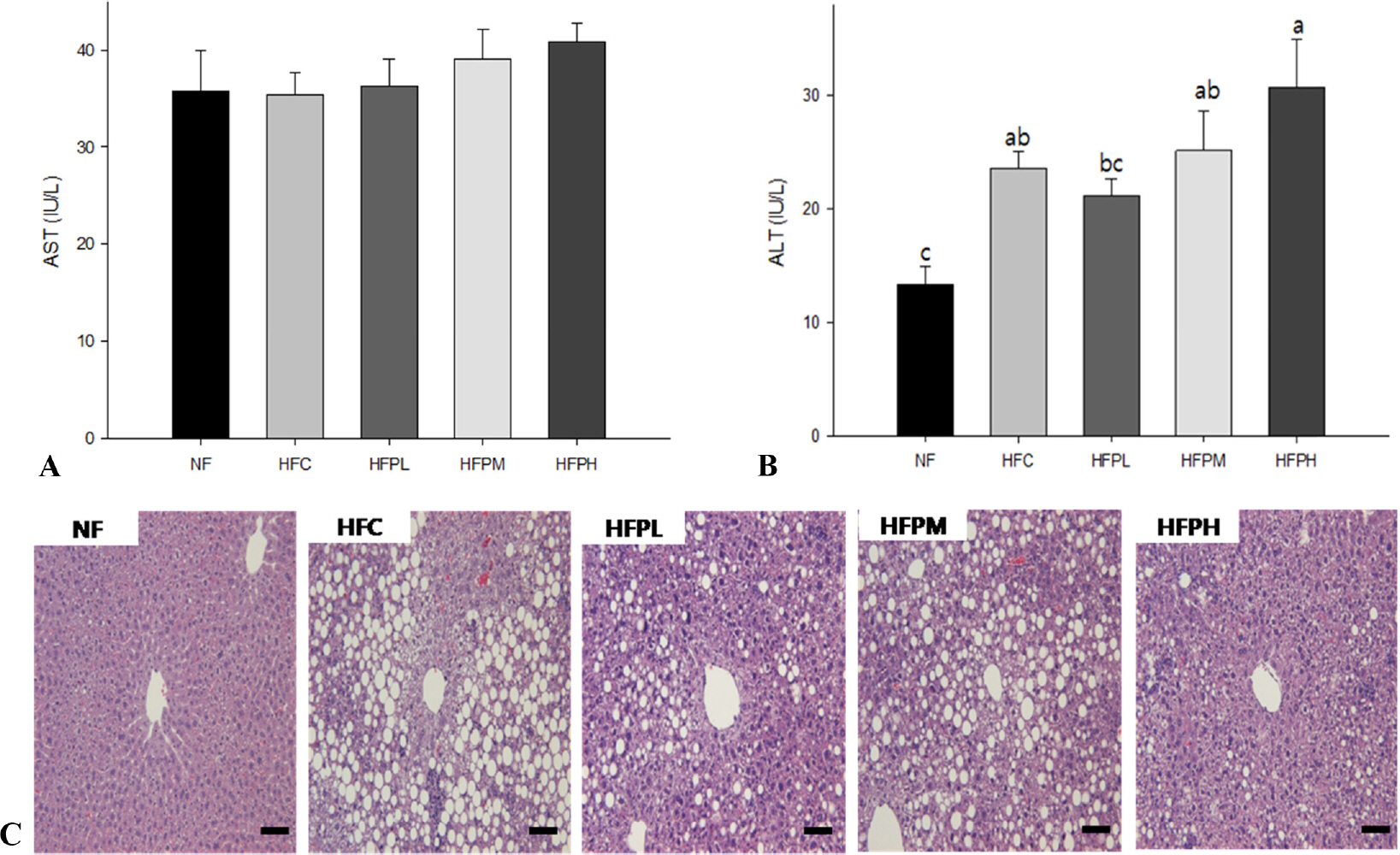
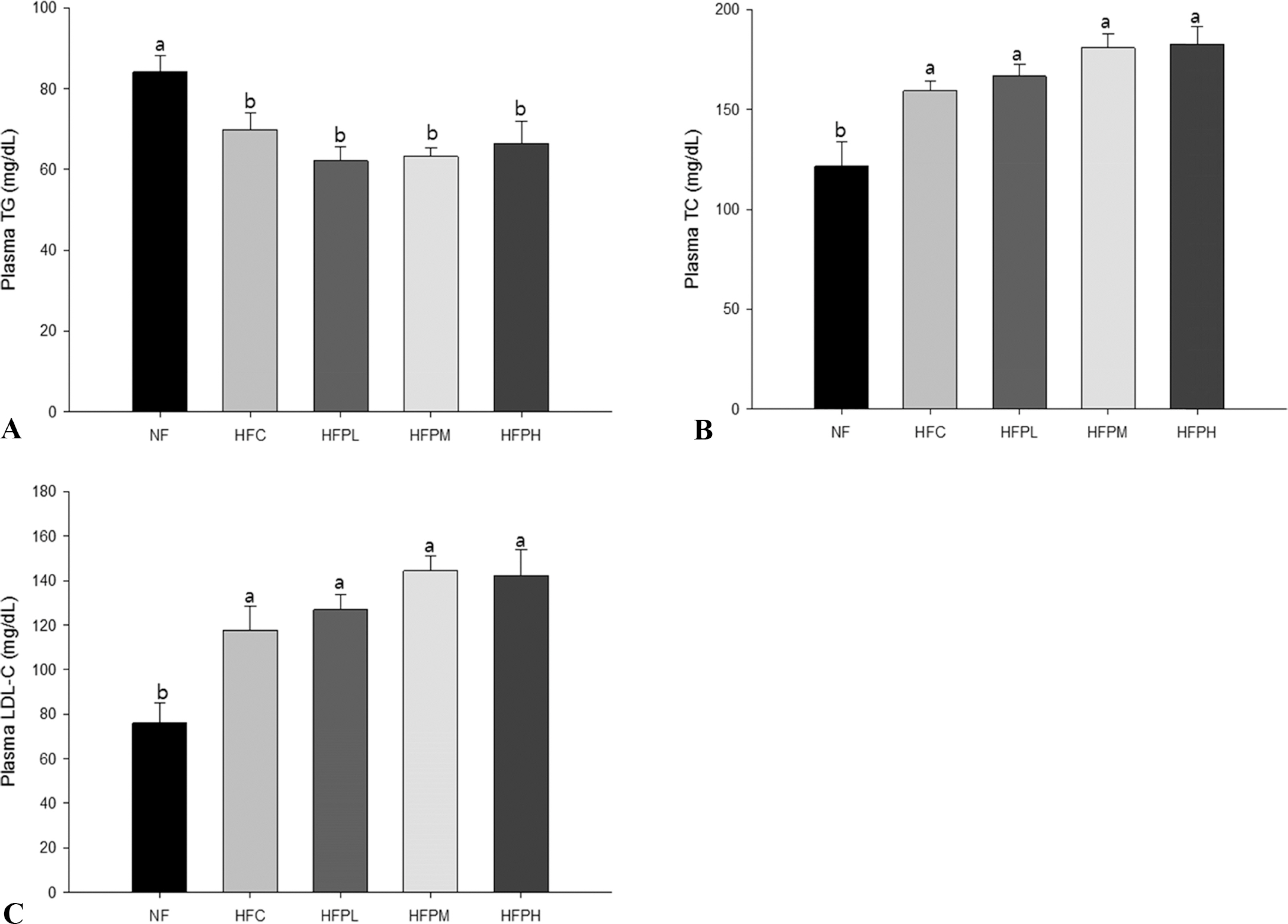
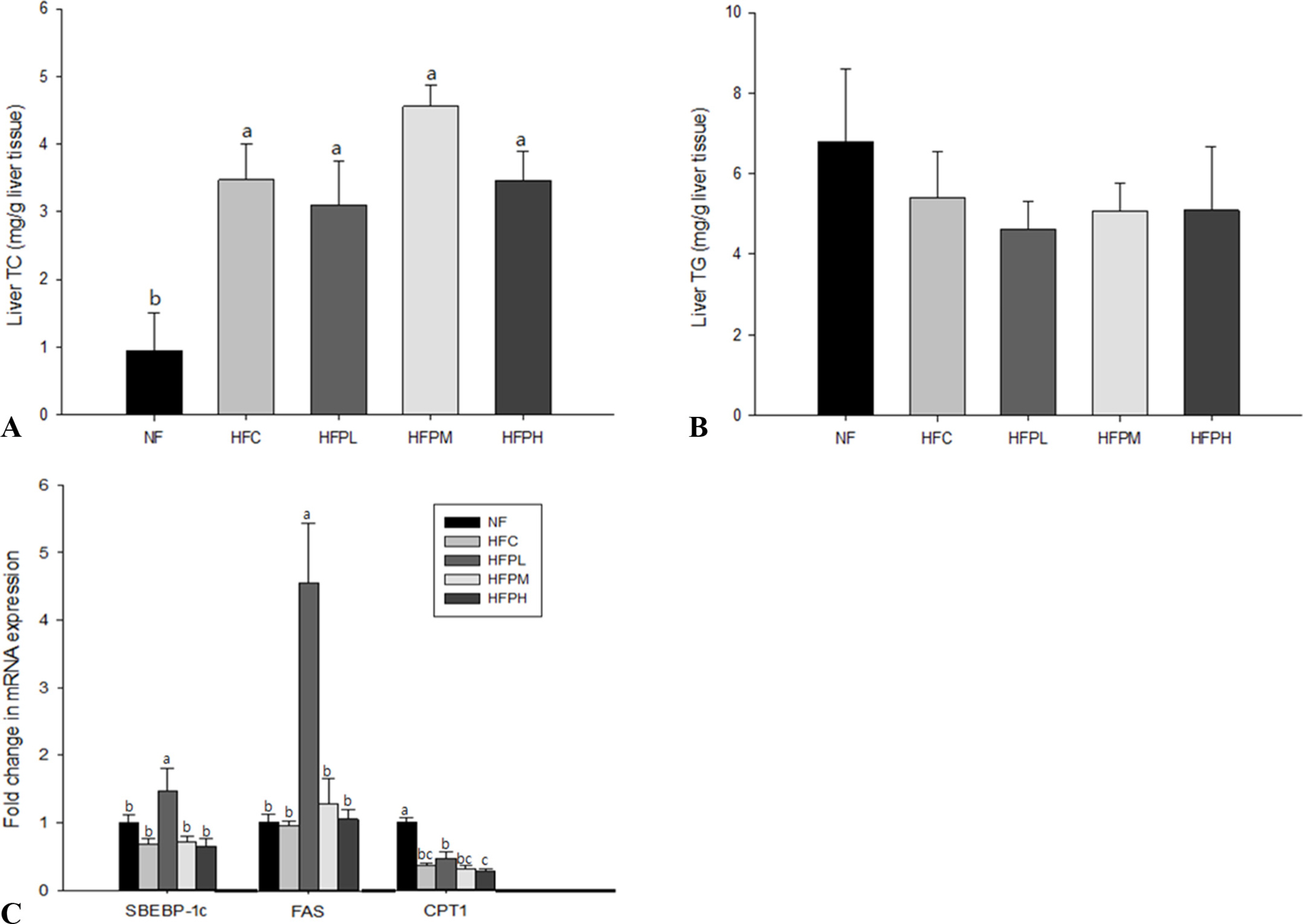
Non-alcoholic fatty liver was manifested in the HFC group by showing increased levels in plasma alanine aminotransferase (ALT) activity, total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C), increased level of TC and presence of many large lipid droplets in the liver, and increased fat cell size in the HFC group compared with the NF group. However, administration of HFC induced a significant decrease in food intake, resulting in decrease in fat mass. Coadministration of PSP did not lead to reversal of body weight changes, ALT activity, and lipid levels in plasma and the liver, but suppressed excess enlargement of the fat cell size through increasing carnitine palmitoyltransferase-1 (CPT-1) gene expression in the liver. Accordingly, the number of fat droplets in the liver was reduced in PSP administered groups.
CONCLUSION
Taken together, these results suggest that PSP may have a protective effect on the dysfunction of lipid metabolism. Conduct of further studies on the coordinated regulation of PSP for lipid metabolic homeostasis at the liver-adipose tissue axis is needed.
MeSH Terms
Figure
Reference
-
1.Samuel VT., Liu ZX., Qu X., Elder BD., Bilz S., Befroy D., Romanelli AJ., Shulman GI. Mechanism of hepatic insulin resistance in nonalcoholic fatty liver disease. J Biol Chem. 2004. 279(31):32345–32353.
Article2.Lee E., Kim WJ., Lee YJ., Lee MK., Kim PG., Park YJ., Kim SK. Effects of natural complex food on specific enzymes of serum and liver and liver microstructure of rats fed a high fat diet. J Korean Soc Food Sci Nutr. 2003. 32(2):256–262.3.Ministry of Food and Drug Safety. Influence of dietary intake on nonalcoholic fatty liver disease in Korean. Cheongwon: Ministry of Food and Drug Safety;2012.4.Hna KH., Lee JC., Kim JH., Lee JS. Manufacture and physiological functionality of Korean traditional liquor by using purple-fleshed sweet potato. Korean J Food Sci Technol. 2002. 34(4):673–677.5.Rossi A., Serraino I., Dugo P., Di Paola R., Mondello L., Genovese T., Morabito D., Dugo G., Sautebin L., Caputi AP., Cuzzocrea S. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic Res. 2003. 37(8):891–900.
Article6.Hwang YP., Choi JH., Han EH., Kim HG., Wee JH., Jung KO., Jung KH., Kwon KI., Jeong TC., Chung YC., Jeong HG. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr Res. 2011. 31(12):896–906.
Article7.Ramirez-Tortosa C., Andersen ØM., Cabrita L., Gardner PT., Morrice PC., Wood SG., Duthie SJ., Collins AR., Duthie GG. Anthocyanin-rich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Radic Biol Med. 2001. 31(9):1033–1037.
Article8.Hwang YP., Choi JH., Yun HJ., Han EH., Kim HG., Kim JY., Park BH., Khanal T., Choi JM., Chung YC., Jeong HG. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem Toxicol. 2011. 49(1):93–99.
Article9.Han KH., Matsumoto A., Shimada K., Sekikawa M., Fukushima M. Effects of anthocyanin-rich purple potato flakes on antioxidant status in F344 rats fed a cholesterol-rich diet. Br J Nutr. 2007. 98(5):914–921.
Article10.Sakatani M., Suda I., Oki T., Kobayashi S., Kobayashi S., Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev. 2007. 53(3):605–614.
Article11.Hwang YP., Choi JH., Choi JM., Chung YC., Jeong HG. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem Toxicol. 2011. 49(9):2081–2089.
Article12.Friedewald WT., Levy RI., Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18(6):499–502.
Article13.Folch J., Lees M., Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226(1):497–509.
Article14.Lovejoy JC. The influence of dietary fat on insulin resistance. Curr Diab Rep. 2002. 2(5):435–440.
Article15.Wildman RP. Healthy obesity. Curr Opin Clin Nutr Metab Care. 2009. 12(4):438–443.16.Siriwardhana N., Kalupahana NS., Cekanova M., LeMieux M., Greer B., Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013. 24(4):613–623.
Article17.Posey KA., Clegg DJ., Printz RL., Byun J., Morton GJ., Vivekanan-dan-Giri A., Pennathur S., Baskin DG., Heinecke JW., Woods SC., Schwartz MW., Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009. 296(5):E1003–E1012.
Article18.Nam KS., Kim J., Noh SK., Park JH., Sung EG. Effect of sweet persimmon wine on alcoholic fatty livers in rats. J Korean Soc Food Sci Nutr. 2011. 40(11):1548–1555.
Article19.Yun TS., Min AK., Kim NK., Kim MK., Cho HC., Kim HS., Hwang JS., Ryu SY., Park KG., Lee IK. Effects of alpha-lipoic acid on SREBP-1c expression in HepG2 cells. J Korean Endocr Soc. 2008. 23(1):27–34.
Article20.Marceau P., Biron S., Hould FS., Marceau S., Simard S., Thung SN., Kral JG. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999. 84(5):1513–1517.
Article21.Bruce CR., Hoy AJ., Turner N., Watt MJ., Allen TL., Carpenter K., Cooney GJ., Febbraio MA., Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009. 58(3):550–558.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cancer-preventive Properties of an Anthocyanin-enriched Sweet Potato in the APCMIN Mouse Model
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet
- Optimization of Brown Rice Cookies using Purple Sweet Potato
- An Activation of Peroxisome Proliferator-Activated Receptor delta Attenuate Alcoholic Liver Disease and Nonalcoholic Fatty Liver Disease in Rats
- Effects of Intermittent Fasting on Splenic Galectin-3 Protein Expression in High-fat Diet-fed Mice