Tuberc Respir Dis.
2014 Mar;76(3):120-126.
Apigenin and Wogonin Regulate Epidermal Growth Factor Receptor Signaling Pathway Involved in MUC5AC Mucin Gene Expression and Production from Cultured Airway Epithelial Cells
- Affiliations
-
- 1Department of Pharmacology, Chungnam National University School of Medicine, Daejeon, Korea. LCJ123@cnu.ac.kr
- 2Pulmonology Section, Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
Abstract
- BACKGROUND
We investigated whether wogonin and apigenin significantly affect the epidermal growth factor receptor (EGFR) signaling pathway involved in MUC5AC mucin gene expression, and production from cultured airway epithelial cells; this was based on our previous report that apigenin and wogonin suppressed MUC5AC mucin gene expression and production from human airway epithelial cells.
METHODS
Confluent NCI-H292 cells were pretreated with wogonin or apigenin for 15 minutes or 24 hours and then stimulated with epidermal growth factor (EGF) for 24 hours or the indicated periods.
RESULTS
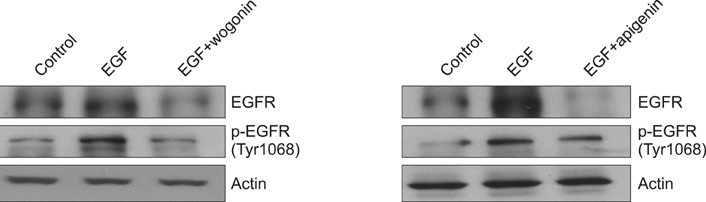
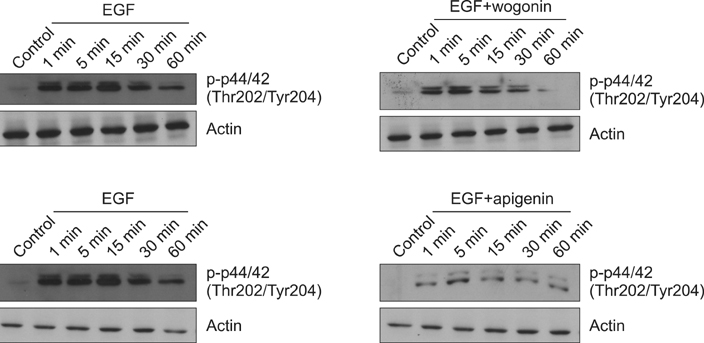
We found that incubation of NCI-H292 cells with wogonin or apigenin inhibited the phosphorylation of EGFR. The downstream signals of EGFR such as phosphorylation of MEK1/2 and ERK1/2 were also inhibited by wogonin or apigenin.
CONCLUSION
The results suggest that wogonin and apigenin inhibits EGFR signaling pathway, which may explain how they inhibit MUC5AC mucin gene expression and production induced by EGF.
Keyword
MeSH Terms
Figure
Reference
-
1. Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med. 1999; 160(5 Pt 2):S44–S48.2. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125.3. Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002; 102:1414–1420.4. Lee HJ, Lee SY, Bae HS, Kim JH, Chang GT, Seok JH, et al. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine. 2011; 18:743–747.5. Lee HJ, Lee SY, Jeon BK, Lee JW, Kim YS, Lee MN, et al. Effect of platycodin D on airway MUC5AC mucin production and gene expression induced by growth factor and proinflammatory factor. Biomol Ther. 2010; 18:294–299.6. Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, et al. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011; 25:1196–1200.7. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009; 23:1458–1461.8. Jang IM. Treatise on Asian herbal medicines. Seoul: Haksul-Pyunsukwan in Research Institute of Natural Products of Seoul National University;2003.9. Clere N, Faure S, Martinez MC, Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem. 2011; 9:62–77.10. Paoletti T, Fallarini S, Gugliesi F, Minassi A, Appendino G, Lombardi G. Anti-inflammatory and vascularprotective properties of 8-prenylapigenin. Eur J Pharmacol. 2009; 620:120–130.11. Zhong Y, Krisanapun C, Lee SH, Nualsanit T, Sams C, Peungvicha P, et al. Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur J Cancer. 2010; 46:3365–3374.12. Zhou Y, Rajabi H, Kufe D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol Pharmacol. 2011; 79:886–893.13. Li RR, Pang LL, Du Q, Shi Y, Dai WJ, Yin KS. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol Immunotoxicol. 2010; 32:364–370.14. Cho J, Lee HK. Wogonin inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Biol Pharm Bull. 2004; 27:1561–1564.15. Chun W, Lee HJ, Kong PJ, Lee GH, Cheong IY, Park H, et al. Synthetic wogonin derivatives suppress lipopolysaccharide-induced nitric oxide production and hydrogen peroxide-induced cytotoxicity. Arch Pharm Res. 2005; 28:216–219.16. Sikder MA, Lee HJ, Mia MZ, Park SH, Ryu J, Kim JH, et al. Inhibition of TNF-alpha-induced MUC5AC mucin gene expression and production by wogonin through the inactivation of NF-kappaB signaling in airway epithelial cells. Phytother Res. 2014; 28:62–68.17. Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, et al. Phorbol ester or epidermal growth-factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012; 26:1784–1788.18. Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998; 157(6 Pt 1):1907–1912.19. Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004; 59:992–996.20. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997; 94:967–972.21. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.22. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.23. Lemmon MA, Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994; 19:459–463.24. Hunter T, Cooper JA. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981; 24:741–752.25. Goldkorn T, Balaban N, Matsukuma K, Chea V, Gould R, Last J, et al. EGF-Receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol. 1998; 19:786–798.26. Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996; 274:1194–1197.27. Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, et al. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997; 390:91–96.28. Greenberg AK, Basu S, Hu J, Yie TA, Tchou-Wong KM, Rom WN, et al. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol. 2002; 26:558–564.29. Kohri K, Ueki IF, Shim JJ, Burgel PR, Oh YM, Tam DC, et al. Pseudomonas aeruginosa induces MUC5AC production via epidermal growth factor receptor. Eur Respir J. 2002; 20:1263–1270.30. Mata M, Sarria B, Buenestado A, Cortijo J, Cerda M, Morcillo EJ. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005; 60:144–152.31. Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003; 4:651–657.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apigenin Inhibits Tumor Necrosis Factor-alpha-Induced Production and Gene Expression of Mucin through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells
- Effects of Cynaroside, Cynarin and Linarin on Secretion, Production and Gene Expression of Airway MUC5AC Mucin in NCI-H292 Cells
- Tussilagone suppressed the production and gene expression of MUC5AC mucin via regulating nuclear factor-kappa B signaling pathway in airway epithelial cells
- Hederacoside C Modulates EGF-Induced MUC5AC Mucin Gene Expression by Regulating the MAPK Signaling Pathway in Human Airway Epithelial Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells