Korean J Physiol Pharmacol.
2018 Nov;22(6):671-677. 10.4196/kjpp.2018.22.6.671.
Tussilagone suppressed the production and gene expression of MUC5AC mucin via regulating nuclear factor-kappa B signaling pathway in airway epithelial cells
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Chungnam National University, Daejeon 35015, Korea. LCJ123@cnu.ac.kr
- 2Smith Liberal Arts College and Department of Addiction Science, Graduate School, Sahmyook University, Seoul 01795, Korea. hjy1213@syu.ac.kr
- KMID: 2430089
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.671
Abstract
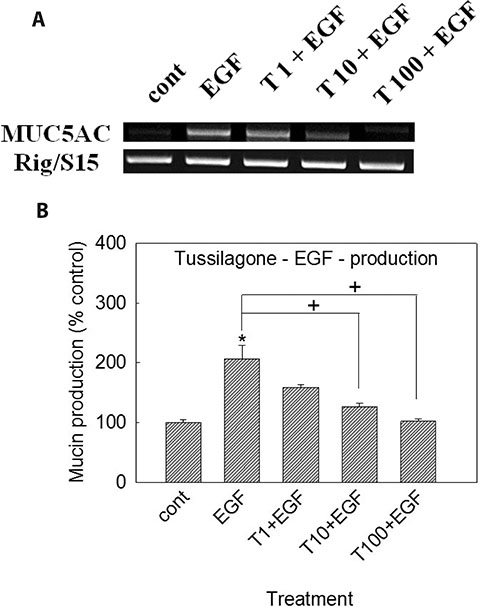
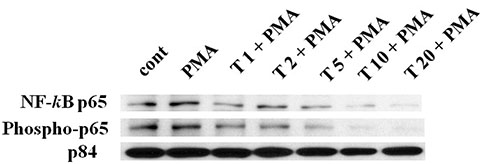
- In the present study, we investigated whether tussilagone, a natural product derived from Tussilago farfara, significantly affects the production and gene expression of airway MUC5AC mucin. Confluent NCI-H292 cells were pretreated with tussilagone for 30 min and then stimulated with EGF (epidermal growth factor) or PMA (phorbol 12-myristate 13-acetate) for 24 h or the indicated periods. The MUC5AC mucin gene expression was measured by RT-PCR. Production of MUC5AC mucin protein was measured by ELISA. To elucidate the action mechanism of tussilagone, effect of tussilagone on PMA-induced NF-κB signaling pathway was investigated by western blot analysis. Tussilagone significantly inhibited the production of MUC5AC mucin protein and down-regulated the expression of MUC5AC mucin gene, induced by EGF or PMA. Tussilagone inhibited PMA-induced activation (phosphorylation) of inhibitory kappa B kinase (IKK), and thus phosphorylation and degradation of inhibitory kappa Ba (IκBα). Tussilagone inhibited PMA-induced phosphorylation and nuclear translocation of nuclear factor kappa B (NF-κB) p65. This, in turn, led to the down-regulation of MUC5AC protein production in NCI-H292 cells. These results suggest that tussilagone can regulate the production and gene expression of mucin by acting on airway epithelial cells through regulation of NF-κB signaling pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Voynow JA, Rubin BK. Mucins, mucus and sputum. Chest. 2009; 135:505–512.
Article2. Lee HJ, Park JS, Yoon YP, Shin YJ, Lee SK, Kim YS, Hong JH, Son KH, Lee CJ. Dioscin and methylprotodioscin isolated from the root of asparagus cochinchinensis suppressed the gene expression and production of airway MUC5AC mucin induced by phorbol ester and growth factor. Phytomedicine. 2015; 22:568–572.
Article3. Kim EJ, Yoon YP, Woo KW, Kim JH, Min SY, Lee HJ, Lee SK, Hong JH, Lee KR, Lee CJ. Verticine, ebeiedine and suchengbeisine isolated from the bulbs of Fritillaria thunbergii Miq. inhibited the gene expression and production of MUC5AC mucin from human airway epithelial cells. Phytomedicine. 2016; 23:95–104.
Article4. Lee HJ, Ryu J, Park SH, Seo EK, Han AR, Lee SK, Kim YS, Hong JH, Seok JH, Lee CJ. Suppressive effects of coixol, glyceryl trilinoleate and natural products derived from Coix Lachryma-Jobi var. Mayuen on gene expression, production and secretion of airway MUC5AC mucin. Arch Pharm Res. 2015; 38:620–627.
Article5. Ryu J, Lee HJ, Park SH, Kim J, Lee D, Lee SK, Kim YS, Hong JH, Seok JH, Lee CJ. Effects of the root of Platycodon grandiflorum on airway mucin hypersecretion in vivo and platycodin D(3) and deapi-platycodin on production and secretion of airway mucin in vitro. Phytomedicine. 2014; 21:529–533.
Article6. Park SH, Lee HJ, Ryu J, Son KH, Kwon SY, Lee SK, Kim YS, Hong JH, Seok JH, Lee CJ. Effects of ophiopogonin D and spicatoside A derived from Liriope tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine. 2014; 21:172–176.
Article7. Lee J, Kang U, Seo EK, Kim YS. Heme oxygenase-1-mediated antiinflammatory effects of tussilagonone on macrophages and 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Int Immunopharmacol. 2016; 34:155–164.8. Hwangbo C, Lee HS, Park J, Choe J, Lee JH. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int Immunopharmacol. 2009; 9:1578–1584.
Article9. Lim HJ, Lee HS, Ryu JH. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression by tussilagone from Farfarae flos in BV-2 microglial cells. Arch Pharm Res. 2008; 31:645–652.
Article10. Kim YK, Yeo MG, Oh BK, Kim HY, Yang HJ, Cho SS, Gil M, Lee KJ. Tussilagone inhibits the inflammatory response and improves survival in CLP-induced septic mice. Int J Mol Sci. 2017; 18.
Article11. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997; 94:967–972.
Article12. Takeyama K, Dabbagh K, Lee HM, Agustí C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.
Article13. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alphaconverting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.14. Ishinaga H, Takeuchi K, Kishioka C, Suzuki S, Basbaum C, Majima Y. Pranlukast inhibits NF-kappaB activation and MUC2 gene expression in cultured human epithelial cells. Pharmacology. 2005; 73:89–96.15. Laos S, Baeckström D, Hansson GC. Inhibition of NF-kappaB activation and chemokine expression by the leukocyte glycoprotein, CD43, in colon cancer cells. Int J Oncol. 2006; 28:695–704.16. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125.
Article17. Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000; 164:1546–1552.
Article18. Hong DH, Petrovics G, Anderson WB, Forstner J, Forstner G. Induction of mucin gene expression in human colonic cell lines by PMA is dependent on PKC-epsilon. Am J Physiol. 1999; 277:G1041–G1047.19. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, REK and Sp1-dependent mechanisms. J Mol Biol. 2004; 344:683–695.20. Park SJ, Kang SY, Kim NS, Kim HM. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol Immunotoxicol. 2002; 24:211–226.
Article21. Kim KD, Lee HJ, Lim SP, Sikder A, Lee SY, Lee CJ. Silibinin regulates gene expression, production and secretion of mucin from cultured airway epithelial cells. Phytother Res. 2012; 26:1301–1307.
Article22. Wu DYC, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol. 2007; 170:20–32.23. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002; 2:725–734.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Apigenin Inhibits Tumor Necrosis Factor-alpha-Induced Production and Gene Expression of Mucin through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways
- Diclofenac Inhibits Phorbol Ester-Induced Gene Expression and Production of MUC5AC Mucin via Affecting Degradation of IkBα and Translocation of NF-kB p65 in NCI-H292 Cells