Korean J Urol.
2010 Feb;51(2):139-144.
The Establishment of Dendritic Cell-Tumor Fusion Vaccines for Hormone Refractory Prostate Cancer Cell
- Affiliations
-
- 1Department of Urology, Gachon University Gil Hospital, Incheon, Korea. urohana@gilhospital.com
- 2Department of Urology, Asan Medical Center, University of Ulsan, Seoul, Korea.
Abstract
-
PURPOSE: Dendritic cell (DC)-based tumor vaccine is an attractive modality for the treatment of hormone-refractory prostate cancer (HRPC) because it has some efficacy and few side effects in patients with poor general conditions. The aim of this study was to establish which is the most effective DC vaccine for the treatment of HRPC. We compared DC vaccine sensitized with tumor lysate and a fusion vaccine of DCs and tumor cells.
MATERIALS AND METHODS
The DU145 cancer cell line was purchased from the American Type Culture Collection. DCs were cultured from peripheral blood monocytes. Peripheral blood monocytes were cultured in RPMI 1640 medium supplemented with interleukin-4 (IL-4), granulocyte-macrophage colony-stimulating factor, and 10% fetal calf serum. Tumor necrosis factor-alpha was added on day 7 to support maturation. Functional activity was measured in three groups: the DC single-culture group, the DC culture group with DC vaccine sensitized with tumor lysates, and the DC culture group prepared with tumor fusion vaccine made from irradiated tumor cells and monocyte-derived DCs by the polyethylene glycol method.
RESULTS
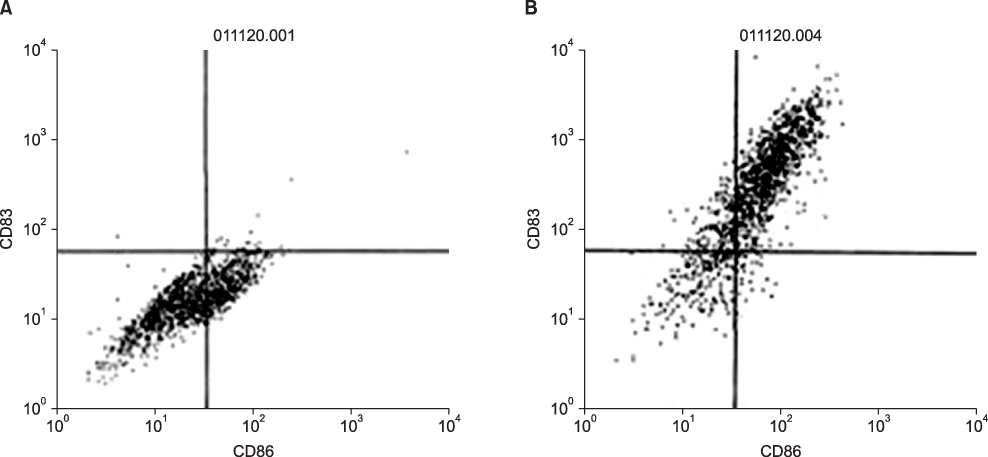
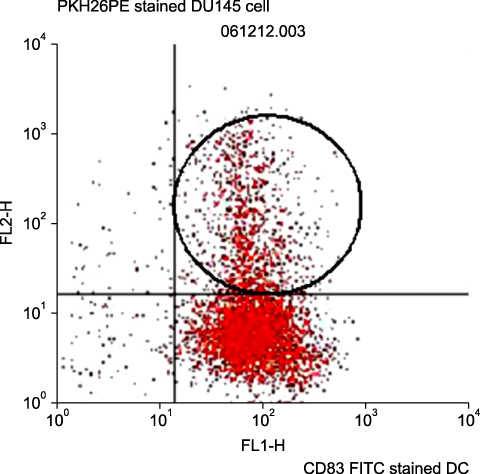
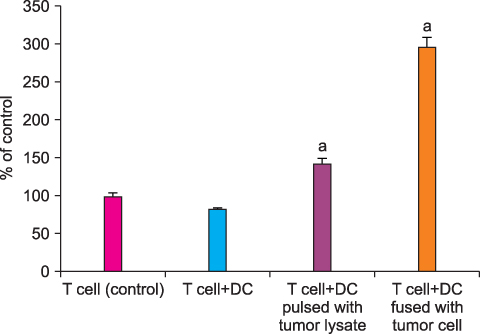
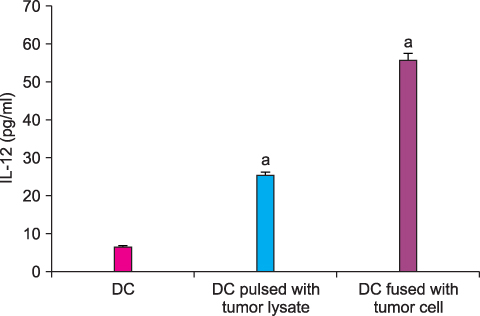
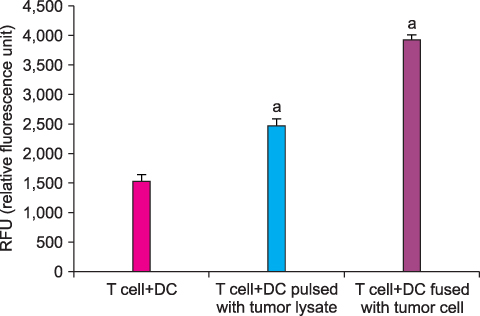
By FACS analysis, the rate of DC-tumor fusion vaccine was 20.3+/-3%. The IL-12 level produced by the DC-tumor fusion vaccine was significantly higher than that of DCs pulsed with tumor lysate (p<0.05). Also, the generation of interferon-gamma by tumor-specific T cells in the DC-tumor fusion vaccine group was superior to that of DCs pulsed with tumor lysate (p<0.05). In addition, the T cells of the tumor lysate-pulsed DCs and tumor fusion vaccine had 1.6 and 2.5 times the functional activity, respectively, of the DC single-culture group in killing tumor cells in the cytotoxicity assay.
CONCLUSIONS
The DC-tumor fusion vaccine seems to be more effective than DC single-culture or DC-tumor lysate vaccine in the treatment of HRPC.
MeSH Terms
-
Cancer Vaccines
Cell Line
Dendritic Cells
Granulocyte-Macrophage Colony-Stimulating Factor
Homicide
Humans
Interferon-gamma
Interleukin-12
Interleukin-4
Monocytes
Polyethylene Glycols
Prostate
Prostatic Neoplasms
T-Lymphocytes
Tumor Necrosis Factor-alpha
Vaccines
Cancer Vaccines
Granulocyte-Macrophage Colony-Stimulating Factor
Interferon-gamma
Interleukin-12
Interleukin-4
Polyethylene Glycols
Tumor Necrosis Factor-alpha
Vaccines
Figure
Reference
-
1. Ha HK, Yun CJ, Lee SS, Shin DG, Lee W, Lee ZZ, et al. Survival rates and related factors in men with hormone-refractory prostate cancer. Korean J Urol. 2009. 50:649–655.2. Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996. 2:52–58.3. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.4. Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998. 4:328–332.5. Kundu SK, Engleman E, Benike C, Shapero MH, Dupuis M, van Schooten WC, et al. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998. 14:551–560.6. Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997. 3:558–561.7. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998. 4:594–600.8. Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996. 29:371–380.9. Segel LA, Jäger E, Elias D, Cohen IR. A quantitative model of autoimmune disease and T-cell vaccination: does more mean less? Immunol Today. 1995. 16:80–84.10. Lotze MT, Shurin M, Davis I, Amoscato A, Storkus WJ. Dendritic cell based therapy of cancer. Adv Exp Med Biol. 1997. 417:551–569.11. Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999. 5:1171–1177.12. Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002. 51:299–310.13. Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007. 117:1195–1203.14. Banchereau J, Steinman RM. Dendritic cells and the control immunity. Nature. 1998. 392:245–252.15. Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992. 149:2681–2688.16. Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996. 184:695–706.17. Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, et al. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta. 2000. 1500:265–279.18. Lundqvist A, Palmborg A, Bidla G, Whelan M, Pandha H, Pisa P. Allogeneic tumor-dendritic cell fusion vaccines for generation of broad prostate cancer T-cell responses. Med Oncol. 2004. 21:155–165.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Approaches in Development of Immunotherapeutic Vaccines for Breast Cancer
- Cancer Vaccines
- Distinct features of dendritic cell-based immunotherapy as cancer vaccines
- Dendritic cell-based therapeutic cancer vaccines: past, present and future
- Efficacy of Dendritic Cells Matured Early with OK-432 (Picibanil(R)), Prostaglandin E2, and Interferon-alpha as a Vaccine for a Hormone Refractory Prostate Cancer Cell Line