Nutr Res Pract.
2015 Feb;9(1):17-21. 10.4162/nrp.2015.9.1.17.
Inhibitory effect of Erythronium japonicum on the human breast cancer cell metastasis
- Affiliations
-
- 1Department of Food & Nutrition, Mokpo National University, 1666, Yeongsan-ro, Cheonggye-myeon, Muan-gun, Jeonnam 534-729, Korea. kha@mokpo.ac.kr
- 2Research Institute of Human Ecology, Mokpo National University, 1666, Yeongsan-ro, Cheonggye-myeon, Muan-gun, Jeonnam 534-729, Korea.
- 3Nature Pure Korea Inc. Jeonnam 517-803, Korea.
- 4Jeonnam Biofood Technology Center, Jeonnam 520-330, Korea.
- KMID: 2313804
- DOI: http://doi.org/10.4162/nrp.2015.9.1.17
Abstract
- BACKGROUND/OBJECTIVES
In this study, the inhibitory effect of Erythronium japonicum extracts on the metastasis of MDA-MB-231 human breast cancer cell line was determined.
MATERIALS/METHODS
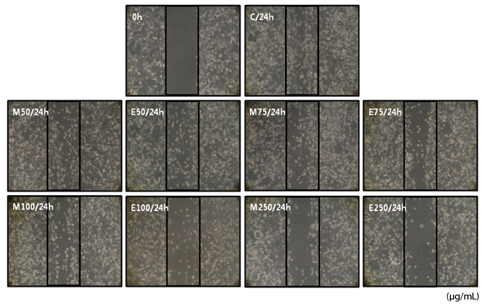
Cells were cultured with DMSO or with 50, 75, 100 or 250 microg/ml of Erythronium japonicum methanol or ethanol extract.
RESULTS
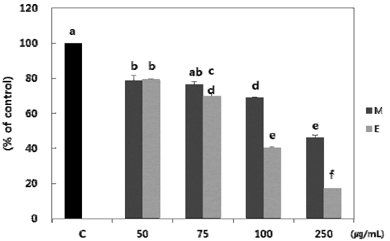
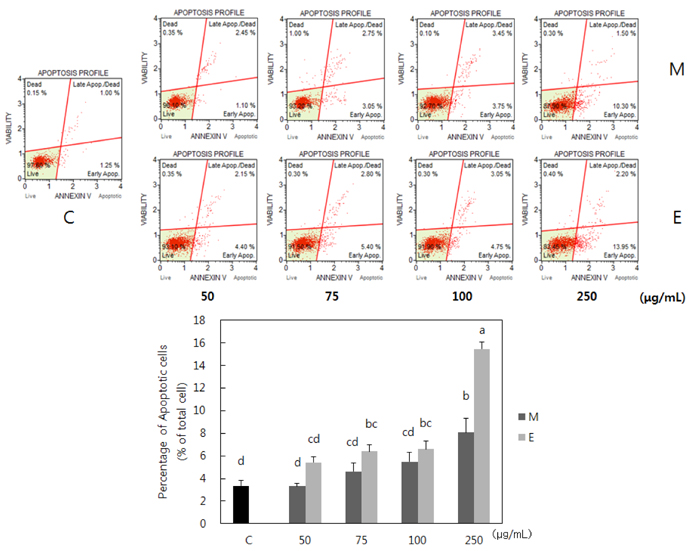
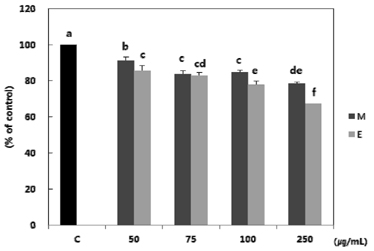
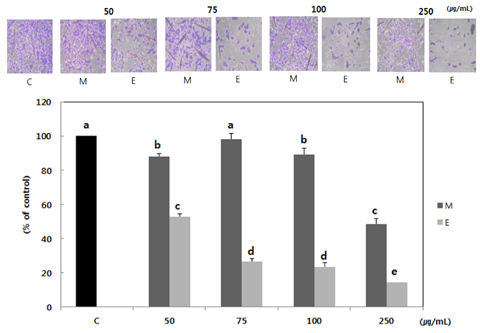
Both methanol and ethanol extracts significantly inhibited the growth and induced apoptosis of MDA-MB-231 cells in a dose-dependent manner. Erythronium japonicum extracts inhibited the adhesion of MDA-MB-231 cells. The invasion of breast cancer cells was suppressed by Erythronium japonicum extracts in a dose-dependent manner. The motility and MMP-2 and MMP-9 activities were also inhibited by both methanol and ethanol extracts.
CONCLUSIONS
Our results collectively indicate that Erythronium japonicum extracts inhibit the growth, adhesion, migration and invasion as well as induce the apoptosis of human breast cancer cells. Clinical application of Erythronium japonicum as a potent chemopreventive agent may be helpful in limiting breast cancer invasion and metastasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000; 21:497–503.
Article2. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003; 3:453–458.
Article3. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001; 411:342–348.
Article4. Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002; 2:277–288.
Article5. Song G, Ohashi T, Sakamoto N, Sato M. Adhesive force of human hepatoma HepG2 cells to endothelial cells and expression of E-selectin. Mol Cell Biomech. 2006; 3:61–68.6. Weber GF. Molecular mechanisms of metastasis. Cancer Lett. 2008; 270:181–190.
Article7. Festuccia C, Guerra F, D'Ascenzo S, Giunciuglio D, Albini A, Bologna M. In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int J Cancer. 1998; 75:418–431.
Article8. Festuccia C, Bologna M, Gravina GL, Guerra F, Angelucci A, Villanova I, Millimaggi D, Teti A. Osteoblast conditioned media contain TGF-beta1 and modulate the migration of prostate tumor cells and their interactions with extracellular matrix components. Int J Cancer. 1999; 81:395–403.
Article9. Kondo T, Okubo N, Miura T, Honda K, Ishikawa Y. Ecophysiology of seed germination in Erythronium japonicum (Liliaceae) with underdeveloped embryos. Am J Bot. 2002; 89:1779–1784.
Article10. Shin YJ, Jung DY, Ha HK, Park SW. Anticancer effect of Erythronium japonicum extract on ICR mouse and L1210 cells with alteration of antioxidant enzyme activities. Korean J Food Sci Technol. 2004; 36:968–973.11. Ritsema T, Smeekens S. Fructans: beneficial for plants and humans. Curr Opin Plant Biol. 2003; 6:223–230.
Article12. Virtanen SS, Väänänen HK, Härkönen PL, Lakkakorpi PT. Alendronate inhibits invasion of PC-3 prostate cancer cells by affecting the mevalonate pathway. Cancer Res. 2002; 62:2708–2714.13. Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF, Chu SC. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J Dent Res. 2006; 85:220–225.
Article14. Goodman SL, Vollmers HP, Birchmeier W. Control of cell locomotion: perturbation with an antibody directed against specific glycoproteins. Cell. 1985; 41:1029–1038.
Article15. Albini A, D'Agostini F, Giunciuglio D, Paglieri I, Balansky R, De Flora S. Inhibition of invasion, gelatinase activity, tumor take and metastasis of malignant cells by N-acetylcysteine. Int J Cancer. 1995; 61:121–129.
Article16. Lee MS, Lim SC, Park HJ. The anthocyanin contents of the petals of Erythronium japonicum collected from several stocks. Korean J Pharmacogn. 1993; 24:251–254.17. Lee MS, Lim SC, Park HJ. Phthalate ester and flavonoids isolated from leaves of Erythronium japonicum. Korean J Med Crop Sci. 1994; 2:67–72.18. Chang WC, Hsieh CH, Hsiao MW, Lin WC, Hung YC, Ye JC. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J Obstet Gynecol. 2010; 49:419–424.
Article19. Hwang HJ, Park HJ, Chung HJ, Min HY, Park EJ, Hong JY, Lee SK. Inhibitory effects of caffeic acid phenethyl ester on cancer cell metastasis mediated by the down-regulation of matrix metalloproteinase expression in human HT1080 fibrosarcoma cells. J Nutr Biochem. 2006; 17:356–362.
Article20. Sanderson JT, Clabault H, Patton C, Lassalle-Claux G, Jean-François J, Paré AF, Hébert MJ, Surette ME, Touaibia M. Antiproliferative, antiandrogenic and cytotoxic effects of novel caffeic acid derivatives in LNCaP human androgen-dependent prostate cancer cells. Bioorg Med Chem. 2013; 21:7182–7193.
Article21. Yamanaka D, Tajima K, Adachi Y, Ishibashi KI, Miura NN, Motoi M, Ohno N. Effect of polymeric caffeic acid on antitumour activity and natural killer cell activity in mice. J Funct Foods. 2014; 6:513–522.
Article22. Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000; 148:779–790.
Article23. Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999; 59:1655–1664.24. Hwang SL, Kim HN, Jung HH, Kim JE, Choi DK, Hur JM, Lee JY, Song H, Song KS, Huh TL. Beneficial effects of β-sitosterol on glucose and lipid metabolism in L6 myotube cells are mediated by AMP-activated protein kinase. Biochem Biophys Res Commun. 2008; 377:1253–1258.
Article25. Kawasaki G, Yanamoto S, Yoshitomi I, Yamada S, Mizuno A. Overexpression of metastasis-associated MTA1 in oral squamous cell carcinomas: correlation with metastasis and invasion. Int J Oral Maxillofac Surg. 2008; 37:1039–1046.
Article26. Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005; 7:128–140.
Article27. Johansson N, Ahonen M, Kähäri VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000; 57:5–15.
Article28. Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010; 15:201–212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- Advances in Clinically Relevant Metastatic Breast Cancer Models
- Effect of Cyanidin on Cell Motility and Invasion in MDA-MB-231 Human Breast Cancer Cells
- Restoration of Hormone Dependency in Estrogen Receptor - Lipofected MDA-MB-231 Human Breast Cancer Cells
- Cytotoxic Triterpenoids from the Fruits of Ligustrum japonicum