Nat Prod Sci.
2018 Jun;24(2):93-98. 10.20307/nps.2018.24.2.93.
Cytotoxic Triterpenoids from the Fruits of Ligustrum japonicum
- Affiliations
-
- 1College of Pharmacy, Drug Research and Development Center, Daegu Catholic University, Gyeongbuk 38430, Republic of Korea. bsmin@cu.ac.kr, weonky@cu.ac.kr
- KMID: 2416068
- DOI: http://doi.org/10.20307/nps.2018.24.2.93
Abstract
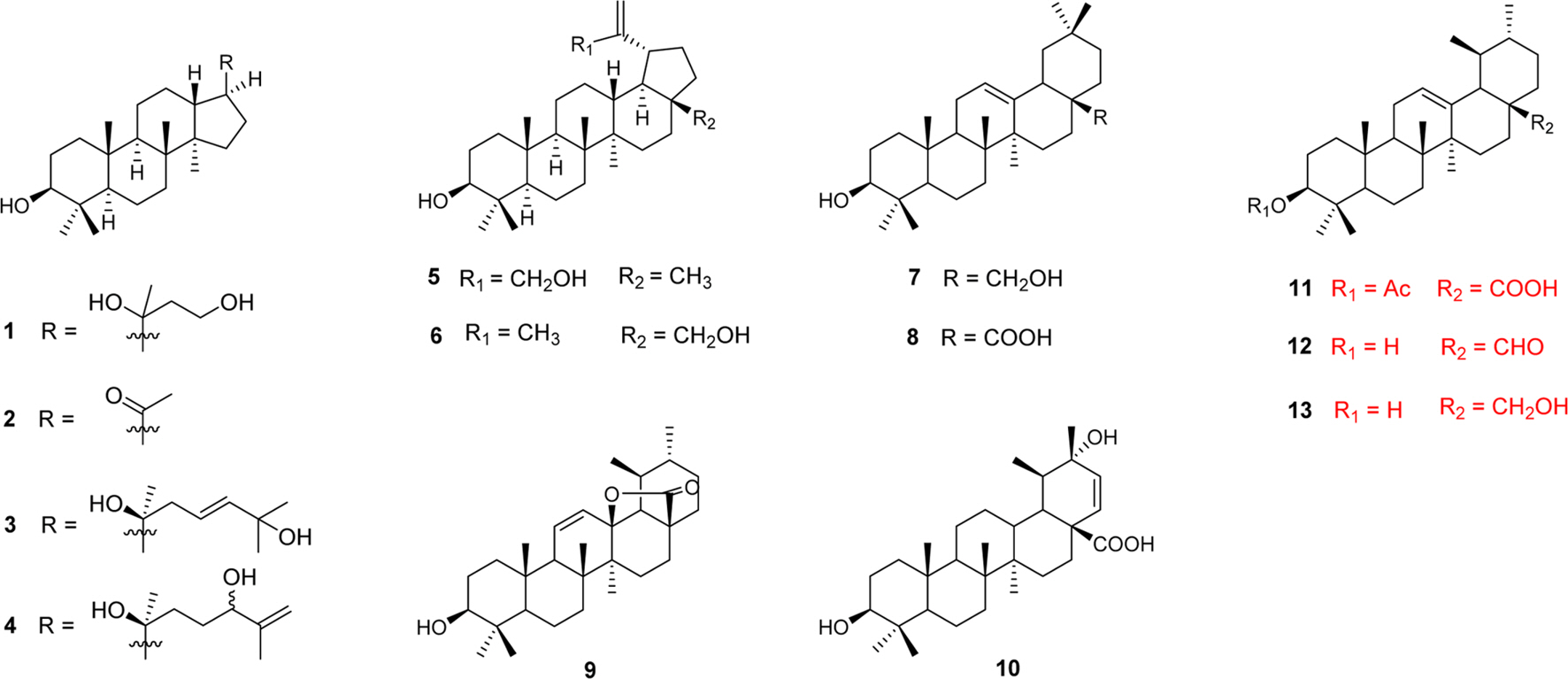
- Medicinal plants are potential sources of anticancer agents screening. A large number of phytochemicals, including triterpenoids, have been reported to have significant cytotoxic effects on cancer cells. From the fruits of Ligustrum japonicum Thunb., thirteen triterpenoids (1 - 13) were isolated and evaluated for their cytotoxic activity against Hela and HL-60 cells. As results, 8 (oleanolic acid) showed significant effects on Hela with IC50 values of 5.5 µM, and moderate effects on HL-60 cells with ICâ‚…â‚€ values of 55.9 µM. Meanwhile, 10 (oleanderic acid) and 11 (3β-acetoxy-urs-12-en-28-oic acid) exhibited moderate inhibitory effects on Hela with ICâ‚…â‚€ value of 55.0 and 68.8 µM, respectively. Moreover, 10 showed cytotoxic effect on HL-60 cell line with ICâ‚…â‚€ value of 63.9 µM. To our knowledge, this is the first report that oleanderic acid was isolated from L. japonicum and investigated in cytotoxic effects on Hela and HL-60 cells.
MeSH Terms
Figure
Reference
-
References
(1). Jensen S. R.., Franzyk H.., Wallander E.Phytochemistry. 2002. 60:213–231.(2). Ngo Q. -M.T.., Lee H. S.., Nguyen V. T.., Kim J. A.., Woo M. H.., Min B. S.Phytochemistry. 2017. 141:147–155.(3). Chudzik M.., Korzonek-Szlacheta I.., Król W.Molecules. 2015. 20:1610–1625.(4). Taher M.., Aminuddin A.., Susanti D.., Aminudin N. I.., On S.., Ahmad F.., Hamidon H.Nat. Prod. Sci. 2016. 22:122–128.(5). Tanaka R.., Matsuda M.., Matsunaga S.Phytochemistry. 1987. 26(3365–3366):. 3365–3366.3365–3366.(6). Pakhathirathien C.., Karalai C.., Ponglimanont C.., Subhadhirasakul S.., Chantrapromma K. J.Nat. Prod. 2005. 68:1787–1789.(7). Chakrabartty T.., Poddar G.., Pyrek J. S.Phytochemistry. 1982. 21:1814–1816.(8). Fuchino H.., Satoh T.., Tanaka N.Chem. Pharm. Bull. 1996. 44:1748–1753. . 1748–1753.(9). Nomura M.., Tokoroyama T.., Kubota T.Phytochemistry. 1981. 20:1097–1104. . 1097–1104.(10). Seebacher W.., Simic N.., Weis R.., Saf R.., Kunert O.Magn. Reson. Chem. 2003. 41:636–638.(11). Al Musayeib N. M.., Mothana R. A.., Gamal A. A. E.., Al-Massarani S. M.., Maes L.Molecules. 2013. 18:9207–9218.(12). Fu L.., Zhang S.., Li N.., Wang J.., Zhao M.., Sakai J.., Hasegawa T.., Mitsui T.., Kataoka T.., Oka S.., Kiuchi M.., Hirose K.., Ando M. J.Nat. Prod. 2005. 68:198–206.(13). Batra A.., Sastry V. G.Pteridines. 2013. 24:191–199.(14). Hota R. K.., Bapuji M.Phytochemistry. 1994. 35:1073–1074.(15). Liao C. R.., Kuo Y. H.., Ho Y. L.., Wang C. Y.., Yang C. S.., Lin C. W.., Chang Y. S.Molecules. 2014. 19:9515–9534.(16). Butruille D.., Dominguez X. A.Tetrahedron Lett. 1974. 15:639–642.(17). Rouf A. S. S.., Ozaki Y.., Rashid M. A.., Rui J.Phytochemistry. 2001. 56:815–818.(18). Zhu Y. Y.., Huang H. Y.., Wu Y. L.Mol. Med. Rep. 2015. 12:5012–5018.(19). Akihisa T.., Kamo S.., Uchiyama T.., Akazawa H.., Banno N.., Taguchi Y.., Yasukawa K. J.Nat. Med. 2006. 60:331–333.(20). Chiang Y. M.., Chang J. Y.., Kuo C. C.., Chang C. Y.., Kuo Y. H.Phytochemistry. 2005. 66:495–501.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic Constituents from the Stem Bark of Chisocheton pentandrus
- Variation of the Contents of Triterpenoids and Tannins Depending on Growth and Infection in the Leaves of Rubus crataegifolius and Rubus parvifolius
- Induction of Apoptosis with Moringa oleifera Fruits in HCT116 Human Colon Cancer Cells Via Intrinsic Pathway
- First Report of Leaf Rust Caused by Puccinia caricis in Farfugium japonicum in Korea
- Generation and characterization of a monoclonal antibody with high species-specificity to Schistosoma japonicum glutathione S-transferase