Nutr Res Pract.
2014 Dec;8(6):618-624. 10.4162/nrp.2014.8.6.618.
Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats
- Affiliations
-
- 1Department of Food and Nutrition, College of Natural Science and Human Ecology, Dong-eui University, 176 Eomgwangno, Busanjin-gu, Busan 614-714, Korea. gayoon@deu.ac.kr
- 2Department of Food and Nutrition, College of Natural Science, Hoseo University, Chungnam 336-795, Korea.
- KMID: 2313787
- DOI: http://doi.org/10.4162/nrp.2014.8.6.618
Abstract
- BACKGROUND/OBJECTIVES
Isoflavones are widely believed to be beneficial to human health, in relation to their antioxidant potentials. Exercise can cause an imbalance between reactive oxygen species (ROS) and antioxidants. This study was conducted in order to investigate the ability of isoflavones in amelioration of oxidative stress induced by exercise.
MATERIALS/METHODS
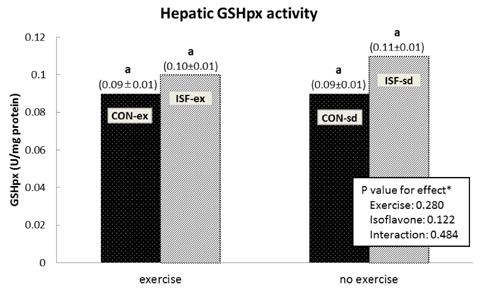
Male Sprague-Dawley rats were assigned to one of four groups: isoflavone-free with no exercise (CON-sd), isoflavone-free with exercise (CON-ex), isoflavone-supplemented with no exercise (ISF-sd), and isoflavone-supplemented with exercise (ISF-ex). Animals exercised on the treadmill for 30 minutes per day, five days per week. TBARS as a marker of oxidative stress and antioxidant enzyme activity, including SOD, GSH-px, and catalase were determined in liver tissue. Serum lipid profile was also examined.
RESULTS
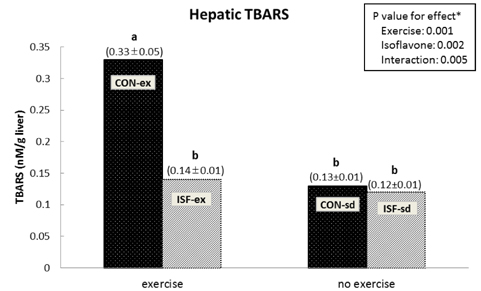
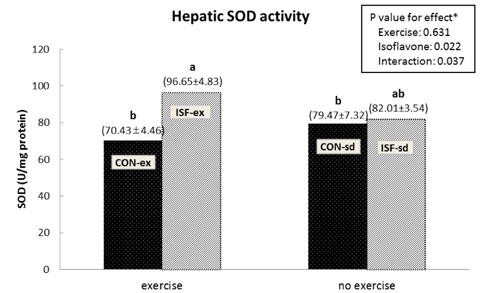
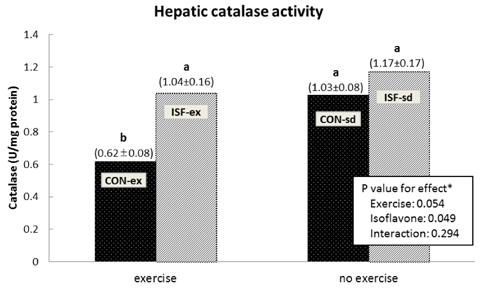
A significant effect of isoflavone alone was observed on abdominal fat pad mass. ISF-ex had significantly less abdominal fat pad than CON-ex. Both exercise and isoflavone treatment had significant effects on lowering plasma triglyceride (TG), thus, the ISF-ex group had a significantly lower TG level than the CON-sd group, by 30.9%. However, no differences were observed in plasma cholesterol, HDL-C, and cholesterol/HDL-C ratio. Exercise, isoflavone, and exercise-isoflavone interaction effects were significant on thiobarbituric acid reactive substances (TBARS) (P = 0.001, 0.002, and 0.005, respectively). The CON-ex group showed a higher TBARS level than the other three groups. By contrast, in the ISF-ex group, TBARS was restored to the level of the ISF-sd or CON-sd group. Isoflavone had a significant effect on superoxide dismutase (SOD) (P = 0.022) and catalase activities (P = 0.049). Significantly higher SOD and catalase activities were observed in ISF-ex than CON-ex. SOD and catalase activities showed an inverse pattern of TBARS. Taken together, isoflavones increased the activities of SOD and catalase with concomitant decreases in TBARS, indicative of decreased oxidative stress.
CONCLUSIONS
Isoflavone supplementation enhances antioxidant action with attenuation of exercise-induced oxidative stress, as measured by decreases in TBARS, and inhibits body fat accumulation and plasma TG increase. Antioxidative effects ascribed to isoflavones may be partially exerted via enhancement of antioxidant enzyme activities.
Keyword
MeSH Terms
-
Abdominal Fat
Adipose Tissue
Animals
Antioxidants
Catalase
Cholesterol
Humans
Isoflavones*
Liver
Male
Oxidative Stress*
Plasma
Rats*
Rats, Sprague-Dawley
Reactive Oxygen Species
Superoxide Dismutase
Thiobarbituric Acid Reactive Substances
Triglycerides
Antioxidants
Catalase
Cholesterol
Isoflavones
Reactive Oxygen Species
Superoxide Dismutase
Thiobarbituric Acid Reactive Substances
Figure
Reference
-
1. Radák Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, Sasvári M, Nyakas C, Goto S. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999; 27:69–74.
Article2. Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001; 131:1010S–1015S.
Article3. Alessio HM, Hagerman AE, Romanello M, Carando S, Threlkeld MS, Rogers J, Dimitrova Y, Muhammed S, Wiley RL. Consumption of green tea protects rats from exercise-induced oxidative stress in kidney and liver. Nutr Res. 2002; 22:1177–1188.
Article4. Yousef MI, Kamel KI, Esmail AM, Baghdadi HH. Antioxidant activities and lipid lowering effects of isoflavone in male rabbits. Food Chem Toxicol. 2004; 42:1497–1503.
Article5. Erba D, Casiraghi MC, Martinez-Conesa C, Goi G, Massaccesi L. Isoflavone supplementation reduces DNA oxidative damage and increases O-β-N-acetyl-D-glucosaminidase activity in healthy women. Nutr Res. 2012; 32:233–240.
Article6. Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys. 2008; 476:102–106.
Article7. Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem. 1999; 47:397–402.
Article8. Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen. 2002; 22:227–234.
Article9. Banz W, Hauck S, Gename B, Winters T, Bartke A. Soy isoflavones modify liver free radical scavenger systems and liver parameters in Sprague-Dawley rats. J Med Food. 2004; 7:477–481.
Article10. Ibrahim WH, Habib HM, Chow CK, Bruckner GG. Isoflavone-rich soy isolate reduces lipid peroxidation in mouse liver. Int J Vitam Nutr Res. 2008; 78:217–222.
Article11. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003; 189:41–54.
Article12. Burneiko RC, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GM, Faine LA, Padovani CR, Cicogna AC, Novelli EL. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006; 44:1167–1172.
Article13. Qiao D, Hou L, Liu X. Influence of intermittent anaerobic exercise on mouse physical endurance and antioxidant components. Br J Sports Med. 2006; 40:214–218.
Article14. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article15. Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984; 105:114–120.16. Aebi H. Catalase. In : Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Academic Press;1974. p. 673–684.17. Fidanza F. Erythrocyte superoxide dismutase. Nutritional status assessment: a manual for population studies. New York (NY): Chapman & Hall;1991. p. 398–401.18. Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007; 18:567–579.
Article19. Chen CY, Bakhiet RM, Hart V, Holtzman G. Isoflavones improve plasma homocysteine status and antioxidant defense system in healthy young men at rest but do not ameliorate oxidative stress induced by 80% VO2pk exercise. Ann Nutr Metab. 2005; 49:33–41.
Article20. Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: a review. Mol Cell Endocrinol. 2009; 304:30–42.
Article21. el-Demerdash FM, Yousef MI, Al-Salhen KS. Protective effects of isoflavone on some biochemical parameters affected by cypermethrin in male rabbits. J Environ Sci Health B. 2003; 38:365–378.
Article22. Weggemans RM, Trautwein EA. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr. 2003; 57:940–946.
Article23. Kawakami Y, Tsurugasaki W, Yoshida Y, Igarashi Y, Nakamura S, Osada K. Regulative actions of dietary soy isoflavone on biological antioxidative system and lipid metabolism in rats. J Agric Food Chem. 2004; 52:1764–1768.
Article24. Demonty I, Lamarche B, Deshaies Y, Jacques H. Role of soy isoflavones in the hypotriglyceridemic effect of soy protein in the rat. J Nutr Biochem. 2002; 13:671–677.
Article25. Sohn E, Deyhim F, Devareddy L, Arjmandi BH. Soy isoflavones attenuate estrogen-deficient-induced increases in abdominal fat in the hamster. Nutr Res. 2004; 24:1023–1029.
Article26. Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med. 2006; 40:341–347.
Article27. Pszczola DE Katz F, Giese J. Research trends in healthful foods. Food Technol. 2000; 54:45–52.28. Castro L, Freeman BA. Reactive oxygen species in human health and disease. Nutrition. 2001; 17:161163–165.
Article29. McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000; 108:652–659.
Article30. Itoh M, Oh-Ishi S, Hatao H, Leeuwenburgh C, Selman C, Ohno H, Kizaki T, Nakamura H, Matsuoka T. Effects of dietary calcium restriction and acute exercise on the antioxidant enzyme system and oxidative stress in rat diaphragm. Am J Physiol Regul Integr Comp Physiol. 2004; 287:R33–R38.
Article31. Oh HY, Lim S, Lee JM, Kim DY, Ann ES, Yoon S. A combination of soy isoflavone supplementation and exercise improves lipid profiles and protects antioxidant defense-systems against exercise-induced oxidative stress in ovariectomized rats. Biofactors. 2007; 29:175–185.
Article32. Hsu CS, Chiu WC, Yeh SL. Effects of soy isoflavone supplementation on plasma glucose, lipids, and antioxidant enzyme activities in streptozotocin-induced diabetic rats. Nutr Res. 2003; 23:67–75.
Article33. Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011; 650:694–702.
Article34. McCarty MF. Isoflavones made simple - genistein's agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses. 2006; 66:1093–1114.
Article35. Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006; 79:1578–1584.
Article36. Dixit AK, Bhatnagar D, Kumar V, Chawla D, Fakhruddin K, Bhatnagar D. Antioxidant potential and radioprotective effect of soy isoflavone against gamma irradiation induced oxidative stress. J Funct Foods. 2012; 4:197–206.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Soy Isoflavone Intake on Nitrite Content and Antioxidant Enzyme Activities in Male Rats Fed High-Fat Diet
- Antioxidant Effect of Garlic Supplement Against Exercise-Induced Oxidative Stress in Rats
- Changes of Antioxidant Enzyme Activities of Rat Brain in the Stress-Related Responses
- Effects of Soy Protein and Isoflavones on Bone Mineral Density in Crowing Female Rats
- Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats