Nutr Res Pract.
2014 Apr;8(2):125-131.
Black rice extract protected HepG2 cells from oxidative stress-induced cell death via ERK1/2 and Akt activation
- Affiliations
-
- 1Department of Food Science and Technology, Chungbuk National University, Cheongju, Chungbuk 361-763, Korea.
- 2National Academy of Agricultural Science, Rural Development Administration, Suwon, Gyeonggi 441-853, Korea.
- 3National Institute of Crop Science, Rural Development Administration, Suwon, Gyeonggi 441-857, Korea.
- 4Department of Horticultural Science, Chungbuk National University, 52 Naesudong-ro, Heungdeok, Cheongju, Chungbuk 361-763, Korea. dkpomo@cbnu.ac.kr
Abstract
- BACKGROUND/OBJECTIVES
The objective of this study was to evaluate the protective effect of black rice extract (BRE) on tert-butyl hydroperoxide (TBHP)-induced oxidative injury in HepG2 cells.
MATERIALS/METHODS
Methanolic extract from black rice was evaluated for the protective effect on TBHP-induced oxidative injury in HepG2 cells. Several biomarkers that modulate cell survival and death including reactive oxygen species (ROS), caspase-3 activity, and related cellular kinases were determined.
RESULTS
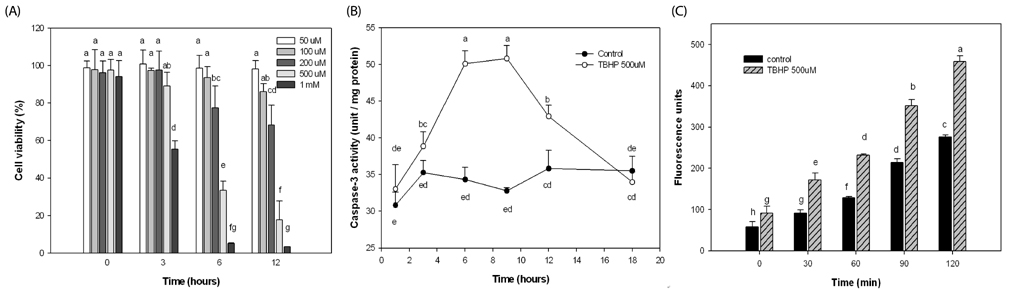
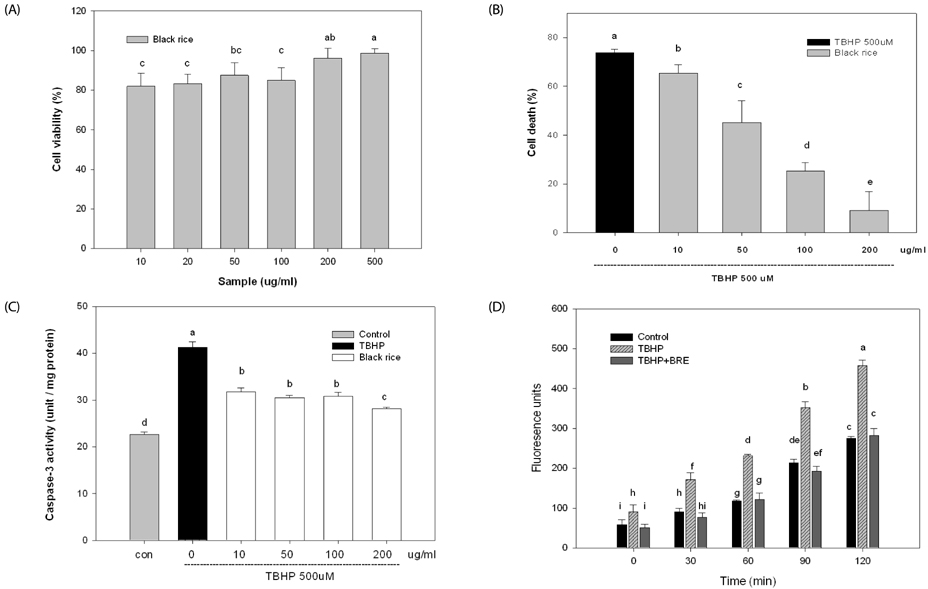
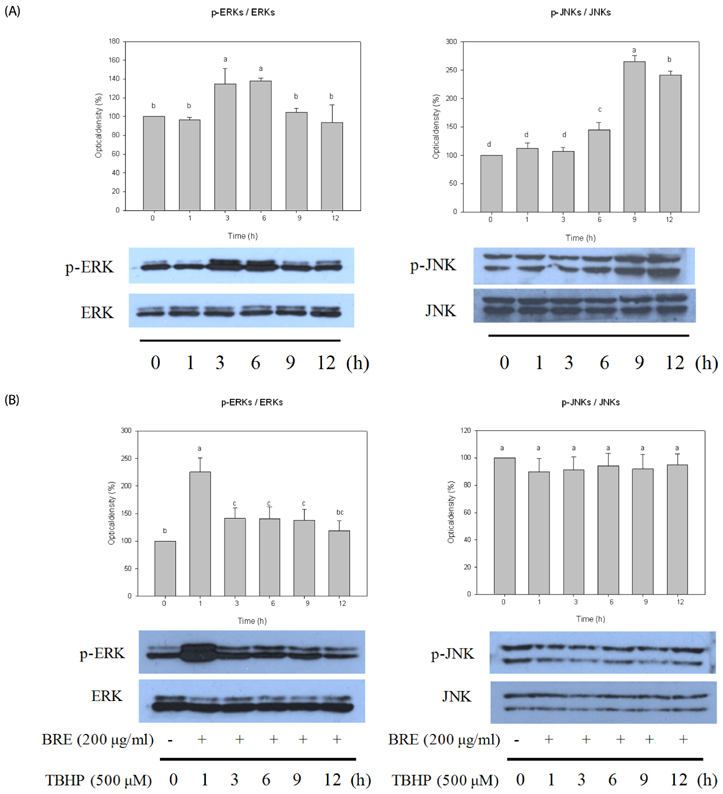
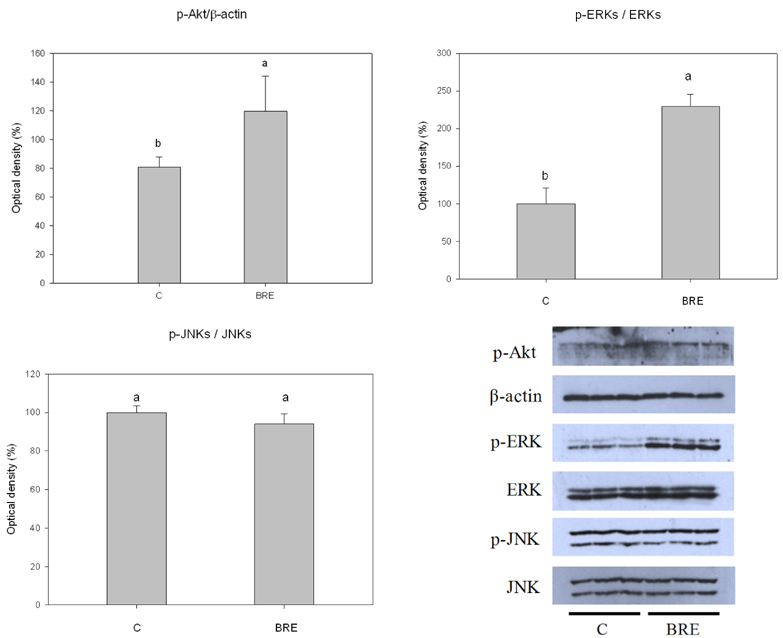
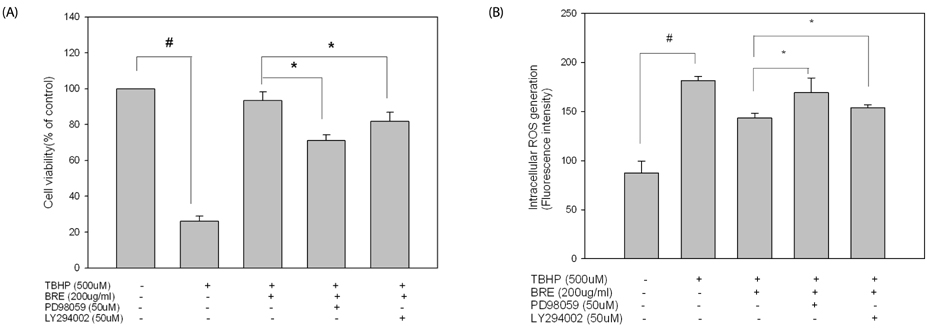
TBHP induced cell death and apoptosis by a rapid increase in ROS generation and caspase-3 activity. Moreover, TBHP-induced oxidative stress resulted in a transient ERK1/2 activation and a sustained increase of JNK1/2 activation. While, BRE pretreatment protects the cells against oxidative stress by reducing cell death, caspase-3 activity, and ROS generation and also by preventing ERKs deactivation and the prolonged JNKs activation. Moreover, pretreatment of BRE increased the activation of ERKs and Akt which are pro-survival signal proteins. However, this effect was blunted in the presence of ERKs and Akt inhibitors.
CONCLUSIONS
These results suggest that activation of ERKs and Akt pathway might be involved in the cytoprotective effect of BRE against oxidative stress. Our findings provide new insights into the cytoprotective effects and its possible mechanism of black rice against oxidative stress.
Keyword
MeSH Terms
Figure
Reference
-
1. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44–84.
Article2. Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006; 72:1439–1452.
Article3. Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005; 16:577–586.
Article4. Chen C, Kong AN. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med. 2004; 36:1505–1516.
Article5. Yang DS, Lee KS, Jeong OY, Kim KJ, Kays SJ. Characterization of volatile aroma compounds in cooked black rice. J Agric Food Chem. 2008; 56:235–240.
Article6. Ling WH, Wang LL, Ma J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J Nutr. 2002; 132:20–26.
Article7. Xia M, Ling WH, Ma J, Kitts DD, Zawistowski J. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein E deficient mice. J Nutr. 2003; 133:744–751.
Article8. Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006; 41:797–803.
Article9. Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010; 10:959–966.
Article10. Guo H, Ling W, Wang Q, Liu C, Hu Y, Xia M. Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-α-induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation. Biochem Pharmacol. 2008; 75:1393–1401.
Article11. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89:271–277.12. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–616.
Article13. Piret JP, Arnould T, Fuks B, Chatelain P, Remacle J, Michiels C. Mitochondria permeability transition-dependent tert-butyl hydroperoxide-induced apoptosis in hepatoma HepG2 cells. Biochem Pharmacol. 2004; 67:611–620.
Article14. Alía M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J Biochem Mol Toxicol. 2005; 19:119–128.
Article15. Mersch-Sundermann V, Knasmüller S, Wu XJ, Darroudi F, Kassie F. Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology. 2004; 198:329–340.
Article16. De Ruvo C, Amodio R, Algeri S, Martelli N, Intilangelo A, D'Ancona GM, Esposito E. Nutritional antioxidants as antidegenerative agents. Int J Dev Neurosci. 2000; 18:359–366.
Article17. Martín MA, Serrano AB, Ramos S, Pulido MI, Bravo L, Goya L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J Nutr Biochem. 2010; 21:196–205.
Article18. Ryu SN, Park SZ, Ho CT. High performance liquid chromatographic determination of anthocyanin pigments in some varieties of black rice. J Food Drug Anal. 1998; 6:729–736.
Article19. Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007; 22:Suppl 1. S45–S48.
Article20. Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007; 8:950–988.
Article21. Zhang B, Kang M, Xie Q, Xu B, Sun C, Chen K, Wu Y. Anthocyanins from Chinese bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. J Agric Food Chem. 2011; 59:537–545.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Cigarette Smoke Extract (CSE)-induced Cell Death in Lung Epithelial Cells
- Water Extract of Samultang Reduces Apoptotic Cell Death by H2O2-Induced Oxidative Injury in SK-N-MC Cells
- Role of Ca2+ influx in the tert-butyl hydroperoxide-induced apoptosis of HepG2 human hepatoblastoma cellse
- Overexpressed Mitochondrial Thioredoxin Protects PC12 Cells from Hydrogen Peroxide and Serum-deprivation
- Oxidative stress and endometriosis