Nutr Res Pract.
2010 Jun;4(3):196-202.
Neuroprotective effects of consuming bovine colostrum after focal brain ischemia/reperfusion injury in rat model
- Affiliations
-
- 1Department of Emergency Medicine, College of Medicine, Kyung Hee University, Seoul 130-702, Korea.
- 2Department of Fermented Food Science, Seoul University of Venture & Information, 37-18 Samsung-dong, Gangnam-gu, Seoul 135-090, Korea. sakang@suv.ac.kr
- 3Department of Food and Nutrition, Kangwon National University, Gangwon 245-711, Korea.
Abstract
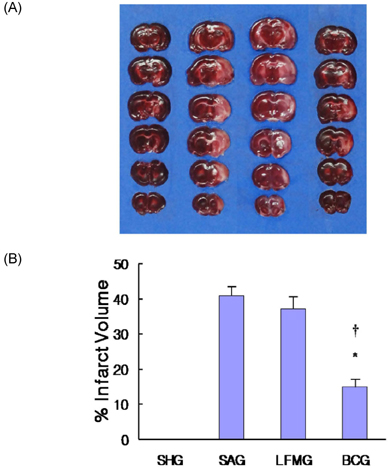
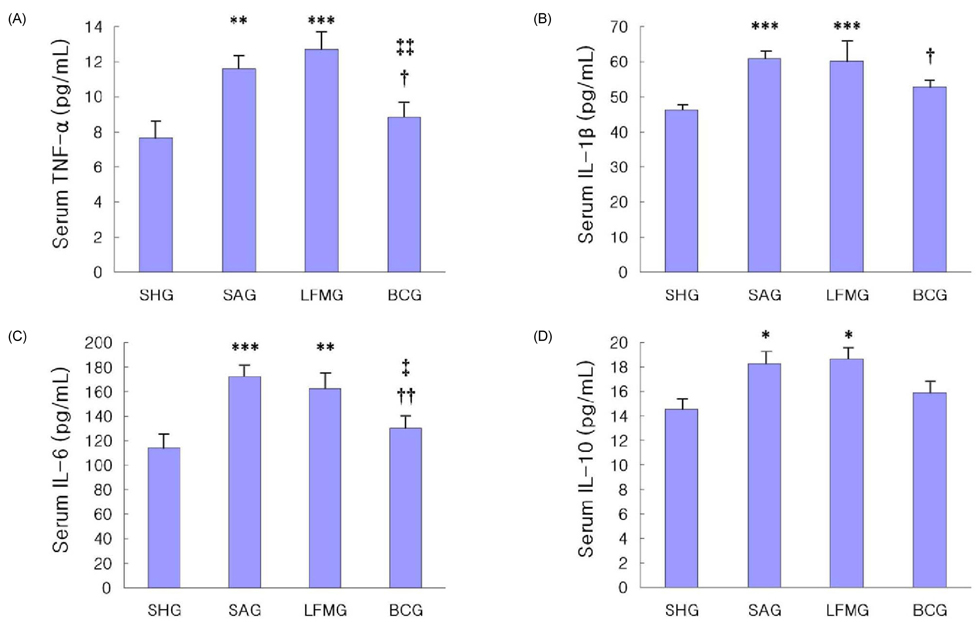
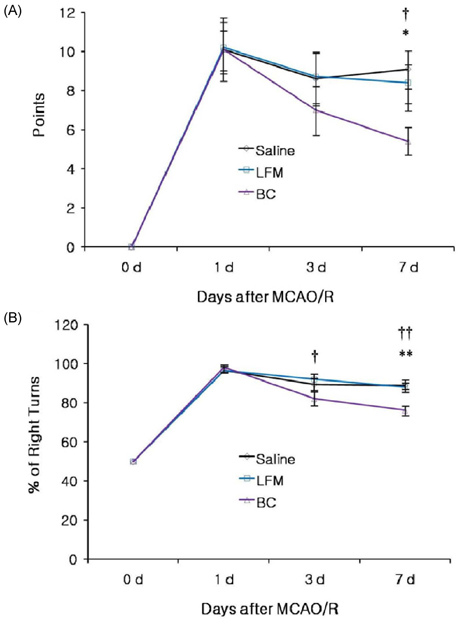
- To investigate the neuroprotective effects of bovine colostrums (BC), we evaluate the ability of consuming BC after focal brain ischemia/reperfusion injury rat model to reduce serum cytokine levels and infarct volume, and improve neurological outcome. Sprague-Dawley rats were randomly divided into 4 groups; one sham operation and three experimental groups. In the experimental groups, MCA occlusion (2 h) and subsequent reperfusion (O/R) were induced with regional cerebral blood flow monitoring. One hour after MCAO/R and once daily during the experiment, the experimental group received BC while the other groups received 0.9% saline or low fat milk (LFM) orally. Seven days later, serum pro-inflammatory cytokine (IL-1beta, IL-6, and TNF-alpha) and anti-inflammatory cytokine (IL-10) levels were assessed. Also, the infarct volume was assessed by using a computerized image analysis system. Behavioral function was also assessed using a modified neurologic severity score and corner turn test during the experiment. Rats receiving BC after focal brain I/R showed a significant reduction (-26%/-22%) in infarct volume compared to LFM/saline rats, respectively (P < 0.05). Serum IL-1beta, IL-6, and TNF-alpha levels were decreased significantly in rats receiving BC compared to LFM/saline rats (P < 0.05). In behavioral tests, daily BC intake showed consistent and significant improvement of neurological deficits for 7 days after MCAO/R. BC ingestion after focal brain ischemia/reperfusion injury may prevent brain injury by reducing serum pro-inflammatory cytokine levels and brain infarct volume in a rat model.
MeSH Terms
Figure
Reference
-
1. Green AR, Shuaib A. Therapeutic strategies for the treatment of stroke. Drug Discov Today. 2006. 11:681–693.
Article2. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003. 2:43–53.
Article3. Plaisier F, Bastide M, Ouk T, Pétrault O, Laprais M, Stolc S, Bordet R. Stobadine-induced hastening of sensorimotor recovery after focal ischemia/reperfusion is associated with cerebrovascular protection. Brain Res. 2008. 1208:240–249.
Article4. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.5. Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol. 1990. 47:1245–1254.
Article6. Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001. 14:89–94.
Article7. Xiao ZY, Sun CK, Xiao XW, Lin YZ, Li S, Ma H, Song GR, Cheng R. Effects of Ginkgo biloba extract against excitotoxicity induced by NMDA receptors and mechanism thereof. Zhonghua Yi Xue Za Zhi. 2006. 86:2479–2484.8. Saleem S, Zhuang H, Biswal S, Christen Y, Doré S. Ginkgo Biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008. 39:3389–3396.
Article9. Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001. 21:2–14.
Article10. Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997. 28:866–872.
Article11. Chun S, Nam M, Goh J, Kim W, Han Y, Kim P. Kinetics and biological function of transforming growth factor-β isoforms in bovine and human colostrum. J Microbiol Biotechnol. 2004. 14:1267–1274.12. Playford RJ. Peptide therapy and the gastroenterologist: colostrum and milk-derived growth factors. Clin Nutr. 2001. 20:101–106.
Article13. Playford RJ, Floyd DN, Macdonald CE, Calnan DP, Adenekan RO, Johnson W, Goodlad RA, Marchbank T. Bovine colostrum is a health food supplement which prevents NSAID induced gut damage. Gut. 1999. 44:653–658.
Article14. Choi HS, Jung KH, Lee SC, Yim SV, Chung JH, Kim YW, Jeon WK, Hong HP, Ko YG, Kim CH, Jang KH, Kang SA. Bovine colostrum prevents bacterial translocation in an intestinal ischemia/ reperfusion-injured rat model. J Med Food. 2009. 12:37–46.
Article15. Sun H, Zhao H, Sharpe GM, Arrick DM, Mayhan WG. Effect of chronic alcohol consumption on brain damage following transient focal ischemia. Brain Res. 2008. 1194:73–80.
Article16. Zhang Y, Wang L, Li J, Wang XL. 2-(1-Hydroxypentyl)-benzoate increases cerebral blood flow and reduces infarct volume in rats model of transient focal cerebral ischemia. J Pharmacol Exp Ther. 2006. 317:973–979.
Article17. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.
Article18. Lourbopoulos A, Karacostas D, Artemis N, Milonas I, Grigoriadis N. Effectiveness of a new modified intraluminal suture for temporary middle cerebral artery occlusion in rats of various weight. J Neurosci Methods. 2008. 173:225–234.
Article19. Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009. 29:1262–1272.
Article20. Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990. 10:290–293.
Article21. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001. 32:1005–1011.
Article22. Haelewyn B, Freret T, Pacary E, Schumann-Bard P, Boulouard M, Bernaudin M, Bouët V. Long-term evaluation of sensorimotor and mnesic behaviour following striatal NMDA-induced unilateral excitotoxic lesion in the mouse. Behav Brain Res. 2007. 178:235–243.
Article23. Lee DH, Ha N, Bu YM, Choi HI, Park YG, Kim YB, Kim MY, Kim H. Neuroprotective effect of Buddleja officinalis extract on transient middle cerebral artery occlusion in rats. Biol Pharm Bull. 2006. 29:1608–1612.
Article24. del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000. 10:95–112.
Article25. Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001. 2:734–744.
Article26. Li TJ, Qiu Y, Mao JQ, Yang PY, Rui YC, Chen WS. Protective effects of Guizhi-Fuling-Capsules on rat brain ischemia/ reperfusion injury. J Pharmacol Sci. 2007. 105:34–40.
Article27. Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009. 130:27–33.
Article28. Dinarello CA. Proinflammatory cytokines. Chest. 2000. 118:503–508.
Article29. Pajkrt D, van der Poll T, Levi M, Cutler DL, Affrime MB, van den Ende A, ten Cate JW, van Deventer SJ. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997. 89:2701–2705.
Article30. Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med. 2005. 33:S468–S471.
Article31. Quigley JD 3rd, Drewry JJ. Nutrient and immunity transfer from cow to calf pre- and post-calving. J Dairy Sci. 1998. 81:2779–2790.
Article32. Kelly GS. Bovine colostrums: a review of clinical uses. Altern Med Rev. 2003. 8:378–394.33. Dávalos A, Ricart W, Gonzalez-Huix F, Soler S, Marrugat J, Molins A, Suñer R, Genís D. Effect of malnutrition after acute stroke on clinical outcome. Stroke. 1996. 27:1028–1032.
Article34. Goto M, Maruyama M, Kitadate K, Kirisawa R, Obata Y, Koiwa M, Iwai H. Detection of nterleukin-1 beta in sera and colostrum of dairy cattle and in sera of neonates. J Vet Med Sci. 1997. 59:437–441.
Article35. Hagiwara K, Kataoka S, Yamanaka H, Kirisawa R, Iwai H. Detection of cytokines in bovine colostrum. Vet Immunol Immunopathol. 2000. 76:183–190.
Article36. Filipp D, Alizadeh-Khiavi K, Richardson C, Palma A, Paredes N, Takeuchi O, Akira S, Julius M. Soluble CD14 enriched in colostrum and milk induces B cell growth and differentiation. Proc Natl Acad Sci U S A. 2001. 98:603–608.
Article37. Hagiwara K, Kitajima K, Yamanaka H, Kirisawa R, Iwai H. Development of a sandwich ELISA assay for measuring bovine soluble type II IL-1 receptor (IL1R2) concentration in serum and milk. Cytokine. 2005. 32:132–136.
Article38. Shing CM, Peake JM, Suzuki K, Jenkins DG, Coombes JS. Bovine colostrum modulates cytokine production in human peripheral blood mononuclear cells stimulated with lipopolysaccharide and phytohemagglutinin. J Interferon Cytokine Res. 2009. 29:37–44.
Article39. An MJ, Cheon JH, Kim SW, Park JJ, Moon CM, Han SY, Kim ES, Kim TI, Kim WH. Bovine colostrum inhibits nuclear factor κB-mediated proinflammatory cytokine expression in intestinal epithelial cells. Nutr Res. 2009. 29:275–280.
Article40. Peroulis N, Kourounakis AP, Yiangou M, Paramythiotis D, Kotzampassi K, Hadjipetrou L. Effects of the novel non-steroidal anti-inflammatory compound [N-(2-Thiolethyl)-2-{2-[N'-(2,6-dichlorophenyl) amino]phenyl}acetamide on cytokines and apoptosis in ischaemic rat brain. Arzneimittelforschung. 2006. 56:688–694.
Article41. Kim KY, Kim MY, Choi HS, Jin BK, Kim SU, Lee YB. Thrombin induces IL-10 production in microglia as a negative feedback regulator of TNF-α release. Neuroreport. 2002. 13:849–852.
Article42. Hayashida K, Kaneko T, Takeuchi T, Shimizu H, Ando K, Harada E. Oral administration of lactoferrin inhibits inflammation and nociception in rat adjuvant-induced arthritis. J Vet Med Sci. 2004. 66:149–154.
Article43. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002. 59:514–523.
Article44. Shohami E, Novikov M. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995. 674:55–62.
Article45. Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002. 117:207–214.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effects of the Green Tea Polyphenol(-)-epigallocatechin Gallate Against Ischemia Reperfusion Injury Induced by Middle Cerebral Artery Occlusion in Rats
- Neuroprotective Effect of Focal Ischemic Preconditioning in Transient Focal Ischemia Animal Model
- The effects of gabapentin pretreatment on brain injury induced by focal cerebral ischemia/reperfusion in the rat
- The Effect of U-74389G and MK-801 on the Size of Brain Infarction in the Transient Focal Ischemia-Reperfusion Rat Model
- Diverse Ischemic Postconditioning Protocols Affect the Infarction Size in Focal Ischemic Stroke