Nutr Res Pract.
2008 Jun;2(2):80-84.
Dehydroepiandrosterone supplement increases malate dehydrogenase activity and decreases NADPH-dependent antioxidant enzyme activity in rat hepatocellular carcinogenesis
- Affiliations
-
- 1Department of Food and Nutrition, College of Human Ecology, Seoul National University, Seoul 151-742, Korea. jwonkim@stanford.edu
- 2Stanford Comprehensive Cancer Center, 269 Campus Drive, CCSR 0137, Stanford University School of Medicine, Stanford, CA, 94305, USA.
- 3Division of Hotel Culinary arts, Hyejeon College, Hongseung, Chungnam 350-702, Korea.
Abstract
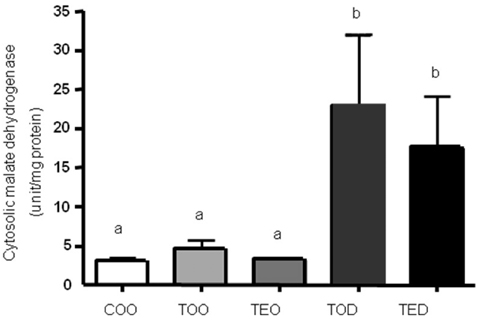
- Beneficial effects of dehydroepiandrosterone (DHEA) supplement on age-associated chronic diseases such as cancer, cardiovascular disease, insulin resistance and diabetes, have been reported. However, its mechanism of action in hepatocellular carcinoma in vivo has not been investigated in detail. We have previously shown that during hepatocellular carcinogenesis, DHEA treatment decreases formation of preneoplastic glutathione S-transferase placental form-positive foci in the liver and has antioxidant effects. Here we aimed to determine the mechanism of actions of DHEA, in comparison to vitamin E, in a chemically-induced hepatocellular carcinoma model in rats. Sprague-Dawley rats were administered with control diet without a carcinogen, diets with 1.5% vitamin E, 0.5% DHEA and both of the compounds with a carcinogen for 6 weeks. The doses were previously reported to have anti-cancer effects in animals without known toxicities. With DHEA treatment, cytosolic malate dehydrogenase activities were significantly increased by ~5 fold and glucose 6-phosphate dehydrogenase activities were decreased by ~25% compared to carcinogen treated group. Activities of Se-glutathione peroxidase in the cytotol was decreased significantly with DHEA treatment, confirming its antioxidative effect. However, liver microsomal cytochrome P-450 content and NADPH-dependent cytochrome P-450 reductase activities were not altered with DHEA treatment. Vitamin E treatment decreased cytosolic Se-glutathione peroxidase activities in accordance with our previous reports. However, vitamin E did not alter glucose 6-phosphate dehydrogenase or malate dehydrogenase activities. Our results suggest that DHEA may have decreased tumor nodule formation and reduced lipid peroxidation as previously reported, possibly by increasing the production of NADPH, a reducing equivalent for NADPH-dependent antioxidant enzymes. DHEA treatment tended to reduce glucose 6-phosphate dehydrogenase activities, which may have resulted in limited supply for de novo synthesis of DNA via inhibiting the hexose monophophaste pathway. Although both DHEA and vitamin E effectively reduced preneoplastic foci in this model, they seemed to function in different mechanisms. In conclusion, DHEA may be used to reduce hepatocellular carcinoma growth by targeting NADPH synthesis, cell proliferation and anti-oxidant enzyme activities during tumor growth.
Keyword
MeSH Terms
-
Animals
Antioxidants
Carcinoma, Hepatocellular
Cardiovascular Diseases
Cell Proliferation
Chronic Disease
Cytochrome P-450 Enzyme System
Cytosol
Dehydroepiandrosterone
Diet
DNA
Glucose
Glutathione Transferase
Insulin Resistance
Lipid Peroxidation
Liver
Malate Dehydrogenase
Malates
NADP
NADPH-Ferrihemoprotein Reductase
Oxidoreductases
Peroxidase
Rats
Rats, Sprague-Dawley
Vitamin E
Vitamins
Antioxidants
Cytochrome P-450 Enzyme System
DNA
Dehydroepiandrosterone
Glucose
Glutathione Transferase
Malate Dehydrogenase
Malates
NADP
NADPH-Ferrihemoprotein Reductase
Oxidoreductases
Peroxidase
Vitamin E
Vitamins
Figure
Reference
-
1. Allolio B, Arlt W, Hahner S. DHEA: Why, when, and how much-DHEA replacement in adrenal insufficiency. Ann Endocrinol (Paris). 2007. 68:268–273.2. Cerutti PA. Prooxidant states and tumor promotion. Science. 1985. 227:375–381.
Article3. Champe PC, Harvey RA. Winters R, Schott J, editors. Hexose monophosphate pathway. Biochemistry. 1987. USA: Lippincott-Raven, Philadelphia, Pennsylvania;111–113.4. Fitzpatrick JL, Ripp SL, Smith NB, Pierce WM, Prough RA Jr. Metabolism of DHEA by cytochromes P450 in rat and human liver microsomal fractions. Arch Biochem Biophys. 2001. 389:278–287.
Article5. Frederiks WM, Van Noordan CJ, Aronson DC, Marx F, Bosch KS, Jonges GN, Vogels IM, James J. Quantitative changes in acid phosphatase, alkaline phosphatase and 5'-nucleotidase activity in rat liver after experimentally induced cholestasis. Liver. 1990. 10:158–166.
Article6. Ito N, Tsuda H, Tatematsu M, Inoue T, Tagawa Y, Aoki T, Uwagawa S, Kagawa M, Ogiso T, Masui T. Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats-an approach for a new medium-term bioassay system. Carcinogenesis. 1988. 9:387–394.
Article7. Kim S, Choi H. Effects of vitamin E and dehydroepiandrosterone on the formation of preneoplastic lesions in rat hepatocellular carcinogenesis. The Korean Journal of Nutrition. 2005. 38:364–372.8. Labrie F. Drug insight: breast cancer prevention and tissue-targeted hormone replacement therapy. Nat Clin Pract Endocrinol Metab. 2007. 3:584–593.
Article9. Ladriere L, Laghmich A, Malaisse-Lagae F, Alaisse WJ. Effect of dehydroepiandrosterone in hereditarily diabetic rat. Cell Biochem Funct. 1997. 15:287–292.10. Lohr GW, Waller HD. Bergmeyer HU, editor. Glucose 6-phosphate dehydrogenase. Methods in enzymatic analysis, (2). 1974. New York. USA: AP.;636–643.11. Marrero M, Prough RA, Frenkel RA, Milewich L. Dehydroepiandrosterone feeding and protein phosphorylation, phosphatases, and lipogenic enzymes in mouse liver. Proc Soc Exp Biol Med. 1990. 193:110–117.
Article12. Muller A, Pallauf J. Effect of increasing selenite concentrations, vitamin E supplementation and different fetal calf serum content on GPx1 activity in primary cultured rabbit hepatocytes. J Trace Elem Med Biol. 2003. 17:183–192.
Article13. Ochoa S. Lowenstein JM, editor. Malic enzyme. Methods in enzymology. 1969. New York, USA: AP.;230–237.14. Omura T, Sato R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I. Evidence for Its Hemoprotein Nature. J Biol Chem. 1964. 239:2370–2378.15. Roberge C, Carpentier C, Langlois M, Baillargeon J, Ardilouze J, Maheux P, Gallo-Payet N. Adrenocortical dysregulation as a major player in insulin resistance and onset of obesity. Am J Physiol Endocrinol Metab. 2007. 293:E1465–E1478.
Article16. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978. 52:506–513.17. Wu HQ, Masset-Brown J, Tweedie DJ, Milewich L, Frenkel RA, Martin-Wixtrom C, Estabrook RW, Prough RA. Induction of microsomal NADPH-cytochrome P-450 reductase and cytochrome P-450IVA1 (P-450LA omega) by dehydroepiandrosterone in rats: a possible peroxisomal proliferator. Cancer Res. 1989. 49:2337–2343.18. Zhao S, Ma H, Zou S, Chen W. Effects of in ovo administration of DHEA on lipid metabolism and hepatic lipogenetic genes expression in broiler chickens during embryonic development. Lipids. 2007. 42:749–757.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of PKCdelta by tyrosine phosphorylation in rat parotid acinar cells
- NADPH Diaphorase Staining Retinal Cells in Streptozotocin-induced Diabetic Rat Retina
- Effects of Vitamin E and Dehydroepiandrosterone on The Formation of Preneoplastic Lesions in Rat Hepatocellular Carcinogenesis
- Lignans with NADPH Oxidase 2 (NOX2)-inhibitory Activity from the Fruits of Schisandra chinensis
- Role of Nitric Oxide in Proximal Urethral Relaxation of the Rat