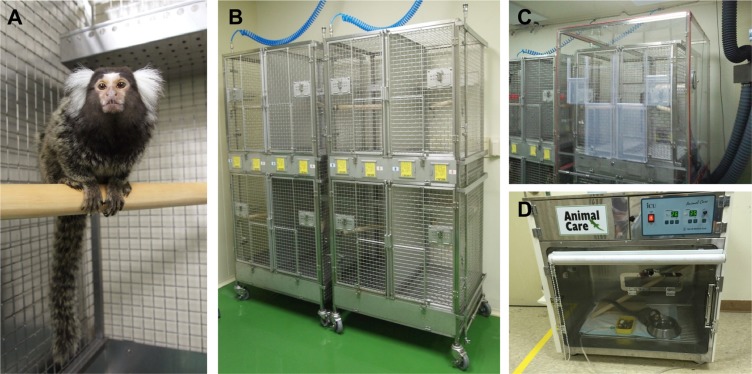
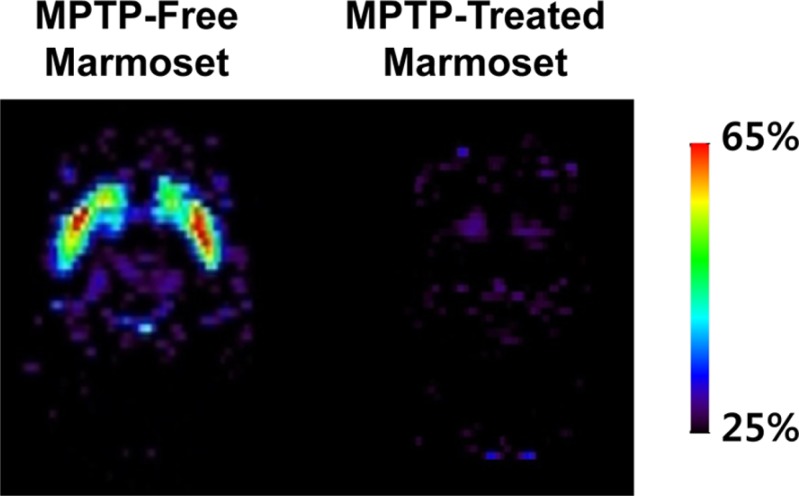
Lab Anim Res.
2015 Dec;31(4):155-165. 10.5625/lar.2015.31.4.155.
Modeling Parkinson's disease in the common marmoset (Callithrix jacchus): overview of models, methods, and animal care
- Affiliations
-
- 1Department of Experimental Animal Research, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea. bckang@snu.ac.kr
- 2Graduate School of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Designed Animal Research Center, Institute of GreenBio Science Technology, Seoul National University, Pyeongchang-gun, Gangwon, Korea.
- KMID: 2312141
- DOI: http://doi.org/10.5625/lar.2015.31.4.155
Abstract
- The common marmoset (Callithrix jacchus) is a small-bodied, popular New World monkey and is used widely in reproductive biology, neuroscience, and drug development, due to its comparative ease of handling, high reproductive efficiency, and its unique behavioral characters. In this review, we discuss the marmoset models in Parkinson's disease (PD), which is a neurological movement disorder primarily resulting from a degeneration of dopaminergic neurons with clinical features of tremor, rigidity, postural instability, and akinesia. The most common PD models involve the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or 6-hydroxydopamine to study the pathogenesis and to evaluate novel therapies. Following the systemic or local administration of these neurotoxins, the marmosets with very severe Parkinson's symptoms are recommended to be placed in an intensive care unit with artificial feeding to increase survival rate. All procedures with MPTP should be conducted in a special room with enclosed cages under negative-pressure by trained researchers with personal protection. Behavioral tests are conducted to provide an external measure of the brain pathology. Along with several biomarkers, including alpha-synuclein and DJ-1, non-invasive neuroimaging techniques such as positron emission tomography and magnetic resonance imaging are used to evaluate the functional changes associated with PD. With the recent growing interest in potential and novel therapies such as stem cell and gene therapy for PD in Korea, the marmoset can be considered as a suitable non-human primate model in PD research to bridge the gap between rodent studies and clinical applications.
Keyword
MeSH Terms
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
alpha-Synuclein
Animals*
Biomarkers
Biology
Brain Diseases
Callithrix*
Dopaminergic Neurons
Genetic Therapy
Humans
Intensive Care Units
Korea
Magnetic Resonance Imaging
Methods*
Models, Animal
Movement Disorders
Neuroimaging
Neurosciences
Neurotoxins
Nutritional Support
Oxidopamine
Parkinson Disease*
Platyrrhini
Positron-Emission Tomography
Primates
Rodentia
Stem Cells
Survival Rate
Tremor
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Neurotoxins
Oxidopamine
alpha-Synuclein
Figure
Reference
-
1. Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998; 339(15):1044–1053. PMID: 9761807.2. Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003; 39(6):889–909. PMID: 12971891.3. Yuan H, Zhang ZW, Liang LW, Shen Q, Wang XD, Ren SM, Ma HJ, Jiao SJ, Liu P. Treatment strategies for Parkinson's disease. Neurosci Bull. 2010; 26(1):66–76. PMID: 20101274.
Article4. Liu Y, Yue F, Tang R, Tao G, Pan X, Zhu L, Kung HF, Chan P. Progressive loss of striatal dopamine terminals in MPTP-induced acute parkinsonism in cynomolgus monkeys using vesicular monoamine transporter type 2 PET imaging ([(18)F]AV-133). Neurosci Bull. 2014; 30(3):409–416. PMID: 24061965.
Article5. Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009; 8(4):382–397. PMID: 19296921.
Article6. Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. In : Yahr M, Bergmann KJ, editors. Parkinson's disease. New York: Raven Press;1987. p. 19–34.7. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004; 318(1):121–134. PMID: 15338272.
Article8. Le W, Sayana P, Jankovic J. Animal models of Parkinson's disease: a gateway to therapeutics? Neurotherapeutics. 2014; 11(1):92–110. PMID: 24158912.
Article9. Blandini F, Armentero MT. Animal models of Parkinson's disease. FEBS J. 2012; 279(7):1156–1166. PMID: 22251459.
Article10. Tieu K. A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harb Perspect Med. 2011; 1(1):a009316. PMID: 22229125.
Article11. Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 2012; 17(6):336–340. PMID: 22871417.
Article12. Orsi A, Rees D, Andreini I, Venturella S, Cinelli S, Oberto G. Overview of the marmoset as a model in nonclinical development of pharmaceutical products. Regul Toxicol Pharmacol. 2011; 59(1):19–27. PMID: 21156195.
Article13. Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus). Comp Med. 2003; 53(4):364–368. PMID: 14524412.14. Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003; 53(4):339–350. PMID: 14524409.15. Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003; 53(4):383–392. PMID: 14524414.16. Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003; 100(7):4078–4083. PMID: 12642658.
Article17. Bezard E, Jaber M, Gonon F, Boireau A, Bloch B, Gross CE. Adaptive changes in the nigrostriatal pathway in response to increased 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration in the mouse. Eur J Neurosci. 2000; 12(8):2892–2900. PMID: 10971632.
Article18. Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998; 152(2):259–277. PMID: 9710526.
Article19. Schneider JS, Schroeder JA, Rothblat DS. Differential recovery of sensorimotor function in GM1 ganglioside-treated vs. spontaneously recovered MPTP-treated cats: partial striatal dopaminergic reinnervation vs. neurochemical compensation. Brain Res. 1998; 813(1):82–87. PMID: 9824674.
Article20. Mikkelsen M, Møller A, Jensen LH, Pedersen A, Harajehi JB, Pakkenberg H. MPTP-induced Parkinsonism in minipigs: A behavioral, biochemical, and histological study. Neurotoxicol Teratol. 1999; 21(2):169–175. PMID: 10192277.21. Baskin DS, Browning JL, Widmayer MA, Zhu ZQ, Grossman RG. Development of a model for Parkinson's disease in sheep using unilateral intracarotid injection of MPTP via slow continuous infusion. Life Sci. 1994; 54(7):471–479. PMID: 8309350.
Article22. Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A, Marsden CD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridineinduced parkinsonism in the common marmoset. Neurosci Lett. 1984; 50(1-3):85–90. PMID: 6436758.
Article23. Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM. Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson's disease. Can J Neurol Sci. 2010; 37(1):86–95. PMID: 20169779.
Article24. Philippens IH, Wubben JA, Finsen B, 't Hart BA. Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J Neuroimmune Pharmacol. 2013; 8(3):715–726. PMID: 23504289.
Article25. Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb Cortex. 2012; 22(2):469–481. PMID: 22114084.
Article26. Smith D, Trennery P, Farningham D, Klapwijk J. The selection of marmoset monkeys (Callithrix jacchus) in pharmaceutical toxicology. Lab Anim. 2001; 35(2):117–130. PMID: 11315160.
Article27. Dell'Mour V, Range F, Huber L. Social learning and mother's behavior in manipulative tasks in infant marmosets. Am J Primatol. 2009; 71(6):503–509. PMID: 19319974.28. Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci. 2005; 25(4):769–777. PMID: 15673656.
Article29. Hansard MJ, Smith LA, Jackson MJ, Cheetham SC, Jenner P. Dopamine, but not norepinephrine or serotonin, reuptake inhibition reverses motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Pharmacol Exp Ther. 2002; 303(3):952–958. PMID: 12438514.
Article30. Annett LE, Torres EM, Clarke DJ, Ishida Y, Barker RA, Ridley RM, Baker HF, Dunnett SB. Survival of nigral grafts within the striatum of marmosets with 6-OHDA lesions depends critically on donor embryo age. Cell Transplant. 1997; 6(6):557–569. PMID: 9440865.
Article31. Bezard E, Przedborski S. A tale on animal models of Parkinson's disease. Mov Disord. 2011; 26(6):993–1002. PMID: 21626544.
Article32. Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, Baker H, Ridley RM, Kirik D. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007; 130(Pt 3):799–815. PMID: 17303591.
Article33. Sasaki E. Prospects for genetically modified non-human primate models, including the common marmoset. Neurosci Res. 2015; 93:110–115. PMID: 25683291.
Article34. Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009; 459(7246):523–527. PMID: 19478777.
Article35. Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010; 66(5):646–661. PMID: 20547124.
Article36. Santana M, Palmér T, Simplício H, Fuentes R, Petersson P. Characterization of long-term motor deficits in the 6-OHDA model of Parkinson's disease in the common marmoset. Behav Brain Res. 2015; 290:90–101. PMID: 25934488.
Article37. Ando K, Obayashi S, Nagai Y, Oh-Nishi A, Minamimoto T, Higuchi M, Inoue T, Itoh T, Suhara T. PET analysis of dopaminergic neurodegeneration in relation to immobility in the MPTP-treated common marmoset, a model for Parkinson's disease. PLoS One. 2012; 7(10):e46371. PMID: 23056291.
Article38. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983; 219(4587):979–980. PMID: 6823561.
Article39. Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979; 1(3):249–254. PMID: 298352.
Article40. Bezard E, Imbert C, Gross CE. Experimental models of Parkinson's disease: from the static to the dynamic. Rev Neurosci. 1998; 9(2):71–90. PMID: 9711900.
Article41. Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001; 76(5):1265–1274. PMID: 11238711.
Article42. Chen MK, Kuwabara H, Zhou Y, Adams RJ, Brasiæ JR, McGlothan JL, Verina T, Burton NC, Alexander M, Kumar A, Wong DF, Guilarte TR. VMAT2 and dopamine neuron loss in a primate model of Parkinson's disease. J Neurochem. 2008; 105(1):78–90. PMID: 17988241.
Article43. Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben-Pazi H, Reches A. Physiology of MPTP tremor. Mov Disord. 1998; 13(Suppl 3):29–34. PMID: 9827591.
Article44. Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonsim and Parkinson's disease. Neuropathologic considerations. Adv Neurol. 1993; 60:600–608. PMID: 8380528.45. Serra PA, Pluchino S, Marchetti B, Desole MS, Miele E. The MPTP mouse model: cues on DA release and neural stem cell restorative role. Parkinsonism Relat Disord. 2008; 14(Suppl 2):S189–S193. PMID: 18579428.
Article46. Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987; 48(6):1787–1793. PMID: 3106573.
Article47. Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985; 36(26):2503–2508. PMID: 2861548.
Article48. Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol. 1986; 20(4):449–455. PMID: 3024555.
Article49. Eslamboli A. Marmoset monkey models of Parkinson's disease: which model, when and why? Brain Res Bull. 2005; 68(3):140–149. PMID: 16325013.
Article50. Fox SH, Henry B, Hill M, Crossman A, Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord. 2002; 17(6):1180–1187. PMID: 12465055.
Article51. Hansard MJ, Smith LA, Jackson MJ, Cheetham SC, Jenner P. Dopamine reuptake inhibition and failure to evoke dyskinesia in MPTP-treated primates. Eur J Pharmacol. 2002; 451(2):157–160. PMID: 12231385.
Article52. van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 2005; 19(9):1140–1142. PMID: 15894565.53. Rose S, Nomoto M, Jackson EA, Gibb WR, Jaehnig P, Jenner P, Marsden CD. Age-related effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment of common marmosets. Eur J Pharmacol. 1993; 230(2):177–185. PMID: 8422900.
Article54. Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007; 2(1):141–151. PMID: 17401348.
Article55. Yang SC, Markey SP, Bankiewicz KS, London WT, Lunn G. Recommended safe practices for using the neurotoxin MPTP in animal experiments. Lab Anim Sci. 1988; 38(5):563–567. PMID: 3264039.56. Tranzer JP, Thoenen H. Selective destruction of adrenergic nerve terminals by chemical analogues of 6-hydroxydopamine. Experientia. 1973; 29(3):314–315. PMID: 4708713.
Article57. Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997; 50:55–66. PMID: 9120425.
Article58. Eslamboli A, Baker HF, Ridley RM, Annett LE. Sensorimotor deficits in a unilateral intrastriatal 6-OHDA partial lesion model of Parkinson's disease in marmoset monkeys. Exp Neurol. 2003; 183(2):418–429. PMID: 14552882.
Article59. Annett LE, Torres EM, Ridley RM, Baker HF, Dunnett SB. A comparison of the behavioural effects of embryonic nigral grafts in the caudate nucleus and in the putamen of marmosets with unilateral 6-OHDA lesions. Exp Brain Res. 1995; 103(3):355–371. PMID: 7789442.
Article60. Henderson JM, Stanic D, Tomas D, Patch J, Horne MK, Bourke D, Finkelstein DI. Postural changes after lesions of the substantia nigra pars reticulata in hemiparkinsonian monkeys. Behav Brain Res. 2005; 160(2):267–276. PMID: 15863223.
Article61. Svenningsson P, Arts J, Gunne L, Andren PE. Acute and repeated treatment with L-DOPA increase c-jun expression in the 6-hydroxydopamine-lesioned forebrain of rats and common marmosets. Brain Res. 2002; 955(1-2):8–15. PMID: 12419516.
Article62. Garea-Rodríguez E, Schlumbohm C, Czéh B, König J, Helms G, Heckmann C, Meller B, Meller J, Fuchs E. Visualizing dopamine transporter integrity with iodine-123-FP-CIT SPECT in combination with high resolution MRI in the brain of the common marmoset monkey. J Neurosci Methods. 2012; 210(2):195–201. PMID: 22827895.
Article63. Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011; 164(4):1357–1391. PMID: 21486284.
Article64. Annett LE, Rogers DC, Hernandez TD, Dunnett SB. Behavioural analysis of unilateral monoamine depletion in the marmoset. Brain. 1992; 115(Pt 3):825–856. PMID: 1352726.
Article65. Mitchell IJ, Hughes N, Carroll CB, Brotchie JM. Reversal of parkinsonian symptoms by intrastriatal and systemic manipulations of excitatory amino acid and dopamine transmission in the bilateral 6-OHDA lesioned marmoset. Behav Pharmacol. 1995; 6(5-6):492–507. PMID: 11224357.
Article66. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000; 3(12):1301–1306. PMID: 11100151.
Article67. Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009; 34(2):279–290. PMID: 19385059.
Article68. Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002; 136(1):317–324. PMID: 12385818.
Article69. Alam M, Schmidt WJ. L-DOPA reverses the hypokinetic behaviour and rigidity in rotenone-treated rats. Behav Brain Res. 2004; 153(2):439–446. PMID: 15265640.
Article70. Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006; 22(2):404–420. PMID: 16439141.71. Emborg ME. Nonhuman primate models of Parkinson's disease. ILAR J. 2007; 48(4):339–355. PMID: 17712221.
Article72. Ando K, Maeda J, Inaji M, Okauchi T, Obayashi S, Higuchi M, Suhara T, Tanioka Y. Neurobehavioral protection by single dose l-deprenyl against MPTP-induced parkinsonism in common marmosets. Psychopharmacology (Berl). 2008; 195(4):509–516. PMID: 17879087.
Article73. Pearce RK, Jackson M, Britton DR, Shiosaki K, Jenner P, Marsden CD. Actions of the D1 agonists A-77636 and A-86929 on locomotion and dyskinesia in MPTP-treated L-dopa-primed common marmosets. Psychopharmacology (Berl). 1999; 142(1):51–60. PMID: 10102782.74. Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994; 14(5 Pt 1):2531–2544. PMID: 8182426.
Article75. Verhave PS, Vanwersch RA, van Helden HP, Smit AB, Philippens IH. Two new test methods to quantify motor deficits in a marmoset model for Parkinson's disease. Behav Brain Res. 2009; 200(1):214–219. PMID: 19378465.
Article76. Przybyszewski AW, Sosale S, Chaudhuri A. Quantification of three-dimensional exploration in the cylinder test by the common marmoset (Callithrix jacchus). Behav Brain Res. 2006; 170(1):62–70. PMID: 16530859.
Article77. Palmér T, Tamtè M, Halje P, Enqvist O, Petersson P. A system for automated tracking of motor components in neurophysiological research. J Neurosci Methods. 2012; 205(2):334–344. PMID: 22306061.
Article78. Philippens IH, Melchers BP, Roeling TA, Bruijnzeel PL. Behavioral test systems in marmoset monkeys. Behav Res Methods Instrum Comput. 2000; 32(1):173–179. PMID: 10758675.
Article79. Annett LE, Martel FL, Rogers DC, Ridley RM, Baker HF, Dunnett SB. Behavioral assessment of the effects of embryonic nigral grafts in marmosets with unilateral 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 1994; 125(2):228–246. PMID: 7906227.
Article80. Henderson JM, Annett LE, Torres EM, Dunnett SB. Behavioural effects of subthalamic nucleus lesions in the hemiparkinsonian marmoset (Callithrix jacchus). Eur J Neurosci. 1998; 10(2):689–698. PMID: 9749730.
Article81. Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, Nomura T, Okano HJ, Kanemura Y, Toyama Y, Okano H. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010; 88(7):1394–1405. PMID: 20091712.
Article82. Montoya CP, Astell S, Dunnett SB. Effects of nigral and striatal grafts on skilled forelimb use in the rat. Prog Brain Res. 1990; 82:459–466. PMID: 2127111.83. Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991; 36(2-3):219–228. PMID: 2062117.
Article84. Blesa J, Juri C, Collantes M, Peñuelas I, Prieto E, Iglesias E, Martí-Climent J, Arbizu J, Zubieta JL, Rodríguez-Oroz MC, García-García D, Richter JA, Cavada C, Obeso JA. Progression of dopaminergic depletion in a model of MPTP-induced Parkinsonism in non-human primates. An (18)F-DOPA and (11)C-DTBZ PET study. Neurobiol Dis. 2010; 38(3):456–463. PMID: 20304066.
Article85. Nagai Y, Minamimoto T, Ando K, Obayashi S, Ito H, Ito N, Suhara T. Correlation between decreased motor activity and dopaminergic degeneration in the ventrolateral putamen in monkeys receiving repeated MPTP administrations: a positron emission tomography study. Neurosci Res. 2012; 73(1):61–67. PMID: 22374309.
Article86. Alexoff DL, Vaska P, Marsteller D, Gerasimov T, Li J, Logan J, Fowler JS, Taintor NB, Thanos PK, Volkow ND. Reproducibility of 11C-raclopride binding in the rat brain measured with the microPET R4: effects of scatter correction and tracer specific activity. J Nucl Med. 2003; 44(5):815–822. PMID: 12732684.87. Collantes M, Prieto E, Peñuelas I, Blesa J, Juri C, Martí-Climent JM, Quincoces G, Arbizu J, Riverol M, Zubieta JL, Rodriguez-Oroz MC, Luquin MR, Richter JA, Obeso JA. New MRI, 18F-DOPA and 11C-(+)-alpha-dihydrotetrabenazine templates for Macaca fascicularis neuroimaging: advantages to improve PET quantification. Neuroimage. 2009; 47(2):533–539. PMID: 19422919.88. Brown CA, Campbell MC, Karimi M, Tabbal SD, Loftin SK, Tian LL, Moerlein SM, Perlmutter JS. Dopamine pathway loss in nucleus accumbens and ventral tegmental area predicts apathetic behavior in MPTP-lesioned monkeys. Exp Neurol. 2012; 236(1):190–197. PMID: 22579525.
Article89. Doudet DJ, Chan GL, Holden JE, McGeer EG, Aigner TA, Wyatt RJ, Ruth TJ. 6-[18F]Fluoro-L-DOPA PET studies of the turnover of dopamine in MPTP-induced parkinsonism in monkeys. Synapse. 1998; 29(3):225–232. PMID: 9635892.
Article90. Lundkvist C, Halldin C, Ginovart N, Swahn CG, Farde L. [18F] beta-CIT-FP is superior to [11C] beta-CIT-FP for quantitation of the dopamine transporter. Nucl Med Biol. 1997; 24(7):621–627. PMID: 9352532.91. Saiki H, Hayashi T, Takahashi R, Takahashi J. Objective and quantitative evaluation of motor function in a monkey model of Parkinson's disease. J Neurosci Methods. 2010; 190(2):198–204. PMID: 20488205.
Article92. Schultz-Darken NJ. Sample collection and restraint techniques used for common marmosets (Callithrix jacchus). Comp Med. 2003; 53(4):360–363. PMID: 14524411.93. Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson's disease. Biochim Biophys Acta. 2009; 1792(7):722–729. PMID: 18992326.
Article94. Miletich RS, Bankiewicz KS, Quarantelli M, Plunkett RJ, Frank J, Kopin IJ, Di Chiro G. MRI detects acute degeneration of the nigrostriatal dopamine system after MPTP exposure in hemiparkinsonian monkeys. Ann Neurol. 1994; 35(6):689–697. PMID: 8210225.
Article95. Stephan H, Baron G, Schwerdtfeger WK. The brain of the common marmoset (Callithrix jacchus): a Stereotaxic Atlas. Berlin: Springer-Verlag;1980.96. Brownell AL, Jenkins BG, Elmaleh DR, Deacon TW, Spealman RD, Isacson O. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nat Med. 1998; 4(11):1308–1312. PMID: 9809556.
Article97. Gröger A, Kolb R, Schäfer R, Klose U. Dopamine reduction in the substantia nigra of Parkinson's disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS One. 2014; 9(1):e84081. PMID: 24416192.
Article98. Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010; 133(Pt 3):713–726. PMID: 20157014.99. Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Leverenz JB, Zhang J. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson's disease. Neurosci Lett. 2010; 480(1):78–82. PMID: 20540987.
Article100. Haavik J, Toska K. Tyrosine hydroxylase and Parkinson's disease. Mol Neurobiol. 1998; 16(3):285–309. PMID: 9626667.
Article101. Barcia C, Ros CM, Annese V, Gómez A, Ros-Bernal F, Aguado-Yera D, Martínez-Pagán ME, de Pablos V, Fernandez-Villalba E, Herrero MT. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis. 2011; 2:e142. PMID: 21472005.
Article102. Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005; 115(1):102–109. PMID: 15630449.
Article103. Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson's disease. J Parkinsons Dis. 2011; 1(4):395–412. PMID: 23933658.
Article104. Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000; 290(5492):767–773. PMID: 11052933.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunolocalization of Aquaporin Water Channels in the Kidney of the Common Marmoset Monkey (Callithrix jacchus)
- Behavior, PET and Histology in Novel Regimen of MPTP Marmoset Model of Parkinson's Disease for Long-Term Stem Cell Therapy
- Immunolocalization of anion exchanger 1 (Band 3) in the renal collecting duct of the common marmoset
- Animal Models of Neurodegenerative Diseases
- Animal models of hemorrhage, parameters, and development of hemostatic methods