J Vet Sci.
2007 Dec;8(4):329-333. 10.4142/jvs.2007.8.4.329.
Immunolocalization of anion exchanger 1 (Band 3) in the renal collecting duct of the common marmoset
- Affiliations
-
- 1Department of Veterinary Medicine and Institute of Veterinary Science, Chungnam National University, Daejeon 305-764, Korea. jyjung@cnu.ac.kr
- 2Department of Anatomy and 3Department of Human Genetics, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- 3Genetic Resources Center, Korea Research Institute of Bioscience & Biotechnology, Daejeon 305-806, Korea.
- KMID: 1106211
- DOI: http://doi.org/10.4142/jvs.2007.8.4.329
Abstract
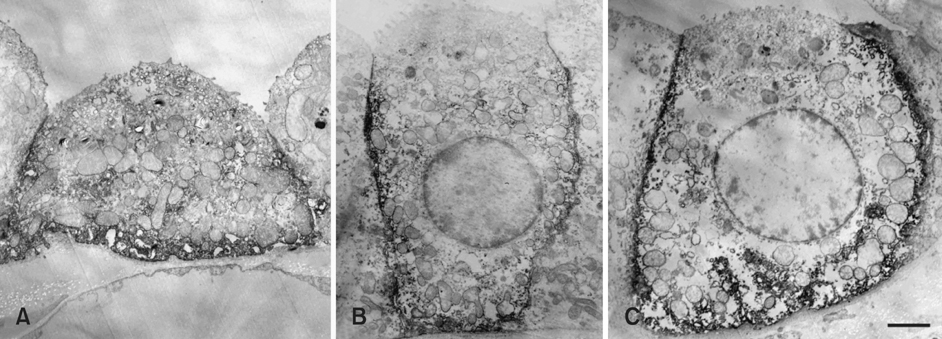
- The purpose of this study was to determine the expression and distribution of band 3 in the collecting duct and connecting tubules of the kidney of the marmoset monkey (Callithrix jacchus), and to establish whether band 3 is expressed in type A intercalated cells. The intracellular localization of band 3 in the different populations of intercalated cells was determined by double-labeling immunohistochemistry. Immunohistochemical microscopy demonstrated that band 3 is located in the basolateral plasma membranes of all type A intercalated cells in the connecting tubule (CNT), cortical collecting duct (CCD), and outer medullary collecting duct (OMCD) of the marmoset. However, type B intercalated cells and non-A/ non-B intercalated cells did not show band 3 labeling. Electron microscopy of the CNT, CCD and OMCD confirmed the light microscopic observation of the basolateral plasma membrane staining for band 3 in a subpopulation of interacted cells. Basolateral staining was seen on the plasma membrane and small coated vesicles in the perinuclear structure, some of which were located in the Golgi region. In addition, there was no labeling of band 3 in the mitochondria of the CNT, CCD and in OMCD cells. The intensity of the immunostaining of the basolateral membrane was less in the CNT than in the CCD and OMCD. In contrast, band 3 immunoreactivity was greater in the intracellular vesicles of the CNT. From these results, we suggest that the basolateral Cl-/HCO3- exchanger in the monkey kidney is in a more active state in the collecting duct than in the CNT.
Keyword
MeSH Terms
-
Animals
Anion Exchange Protein 1, Erythrocyte/*metabolism
Callithrix/*metabolism
Gene Expression Profiling/veterinary
*Gene Expression Regulation
Immunohistochemistry/veterinary
Kidney Tubules/cytology/physiology/ultrastructure
Kidney Tubules, Collecting/cytology/*metabolism/ultrastructure
Male
Microscopy, Electron, Transmission/veterinary
Figure
Reference
-
1. Alper SL, Darman RB, Chernova MN, Dahl NK. The AE gene family of Cl/HCO3- exchangers. J Nephrol. 2002. 5:S41–S53.2. Drenckhahn D, Schlüter K, Allen D, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985. 230:1287–1289.
Article3. Ercolani L, Brown D, Stuart-Tilley A, Alper SL. Colocalization of GAPDH and band 3 (AE1) proteins in rat erythrocytes and kidney intercalated cell membranes. Am J Physiol. 1992. 262:F892–F896.
Article4. Kim J, Tisher CC, Linser PJ, Madsen KM. Ultrastructural localization of carbonic anhydrase II in subpopulations of intercalated cells of the rat kidney. J Am Soc Nephrol. 1990. 1:245–256.
Article5. Kim J, Welch WJ, Cannon JK, Tisher CC, Madsen KM. Immunocytochemical response of type A and type B intercalated cells to increased sodium chloride delivery. Am J Physiol. 1992. 262:F288–F302.
Article6. Kopito RR. Molecular biology of the anion exchanger gene family. Int Rev Cytol. 1990. 123:177–199.
Article7. Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990. 265:462–471.
Article8. Madsen KM, Brenner BM. Tisher CC, Brenner BM, editors. Structure and function of the renal tubule and the interstitium. Renal Pathology with Clinical and Functional Correlations. 1994. Philadelphia: Lippincott;661–698.9. Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl. 1991. 33:S57–S63.10. Madsen KM, Kim J, Tisher CC. Intracellular band 3 immunostaining in type A intercalated cells of rabbit kidney. Am J Physiol. 1992. 262:F1015–F1022.
Article11. Muto S, Yasoshima K, Yoshitomi K, Imai M, Asano Y. Electrophysiological identification of α- and β-intercalated cells and their distribution along the rabbit distal nephron segments. J Clin Invest. 1990. 86:1829–1839.
Article12. Nissant A, Paulais M, Lachheb S, Lourdel S, Teulon J. Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. Am J Physiol Renal Physiol. 2006. 290:F1421–F1429.
Article13. Ostedgaard LS, Jennings ML, Karniski LP, Schuster VL. A 45-kDa protein antigenically related to band 3 is selectively expressed in kidney mitochondria. Proc Natl Acad Sci USA. 1991. 88:981–985.
Article14. Schuster VL, Fejes-Toth C, Naray-Fejes-Toth A, Gluck SL. Colocalization of H+-ATPase and band 3 anion exchanger in rabbit collecting duct intercalated cells. Am J Physiol Renal Physiol. 1991. 260:F506–F517.15. Tisher CC, Madsen KM. Brenner BM, Rector FC, editors. Anatomy of the kidney. The Kidney. 1991. 4th ed. Saunders: New York;3–75.16. Verlander JW, Madsen KM, Tisher CC. Axial distribution of band 3-positive intercalated cells in the collecting duct of control and ammonium chloride-loaded rabbits. Kidney Int Suppl. 1996. 57:S137–S147.17. Verlander JW, Madsen KM, Low PS, Allen DP, Tisher CC. Immunocytochemical localization of band 3 protein in the rat collecting duct. Am J Physiol Renal Physiol. 1988. 255:F115–F125.
Article18. Wagner S, Vogel R, Lietzke R, Koob R, Drenckhahn D. Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol Renal Physiol. 1987. 253:F213–F221.
Article19. Weiner ID, Hamm LL. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest. 1990. 85:274–281.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunolocalization of Aquaporin Water Channels in the Kidney of the Common Marmoset Monkey (Callithrix jacchus)
- Expression of an Anion Exchanger Pendrin in Human Kidney
- A Case of Collecting Duct Carcinoma of Kidney
- Collecting Duct Carcinoma (Bellini Duct Carcinoma) Presented as a Renal Cyst
- A case of collecting duct carcinoma of the kidney