Korean J Urol.
2009 May;50(5):493-497.
The Effects of Adiponectin and Leptin in the Proliferation of Prostate Cancer Cells
- Affiliations
-
- 1Department of Urology, College of Medicine, Chung-Ang University, Seoul, Korea. kthlmk@hanafos.com
Abstract
-
PURPOSE: With the westernization of dietary life, domestic prostate cancer prevalence has remarkably increased recently. Therefore, to examine the effects of obesity on prostate cancer, we analyzed the effects of leptin and adiponectin, which are the cytokines secreted from adipocytes, on prostate cancer in vitro and confirmed the results by in vivo experiment.
MATERIALS AND METHODS
In vitro, the human androgen-independent prostate cancer cell line DU-145 was exposed to various concentrations of adiponectin and leptin, and their effects were measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In vivo, the effects of tumor growth were observed in xenografted nude mice with prostate cancer.
RESULTS
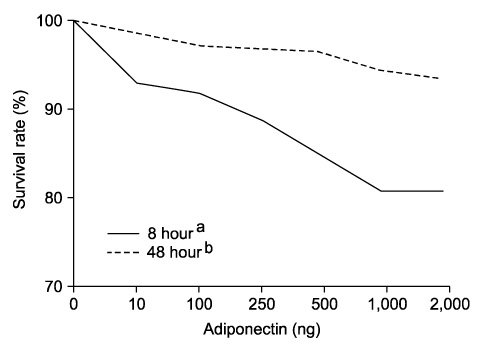
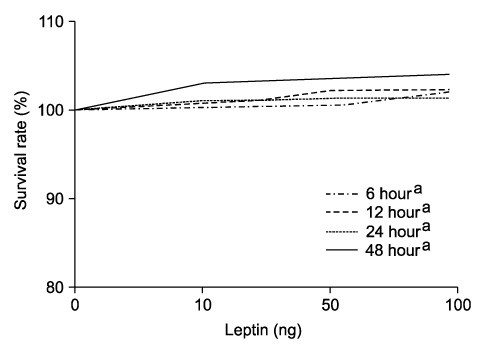
Adiponectin significantly repressed DU-145 cell growth in a dose-dependent manner. Leptin promoted DU-145 cell growth in dose-dependent manner, but it was not significant statistically. In vivo, adiponectin-treated mice demonstrated a reduced tumor volume, although it was not significant statistically. By contrast, leptin-treated mice showed a significantly increased tumor volume (p<0.01).
CONCLUSIONS
The in vitro and in vivo finding suggested that adiponectin suppresses the proliferation of prostate cancer cells and that leptin plays an important role in the proliferation of prostate cancer cells. We suggest that adiponectin and leptin have a relation to the progression of prostate cancer in the obese population.
Keyword
MeSH Terms
Figure
Reference
-
1. Song K, Song C, Ahn H. Continuing trends of the clinical parameter migration in patients with prostate cnacer in Korea. Korean J Urol. 2007. 48:574–578.2. Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006. 340:1158–1166.3. Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002. 277:25863–25866.4. Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002. 106:2767–2770.5. Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998. 33:1055–1059.6. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004. 89:2548–2556.7. Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adioponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007. 86:s858–s866.8. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996. 334:292–295.9. Chang S, Hursting SD, Contois JH, Strom SS, Yamamura Y, Babaian RJ, et al. Leptin and prostate cancer. Prostate. 2001. 46:62–67.10. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999. 257:79–83.11. Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005. 65:1168–1172.12. Cao Y, O'Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J Clin Invest. 1998. 101:1055–1063.13. Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James RG, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA. 2000. 97:14478–14483.14. Takahashi M, Arita Y, Yamagata K, Matsukawa Y, Okutomi K, Horie M, et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord. 2000. 24:861–868.15. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.16. Nazian SJ, Cameron DF. Temporal relation between leptin and various indices of sexual maturation in the male rat. J Androl. 1999. 20:487–491.17. Blum WF, Englaro P, Attanasio AM, Kiess W, Rascher W. Human and clinical perspectives on leptin. Proc Nutr Soc. 1998. 57:477–485.18. Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun. 2005. 333:79–87.19. Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006. 345:271–279.20. Cong L, Gasser J, Zhao J, Yang B, Li F, Zhao AZ. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95 2. Endocr Relat Cancer. 2007. 14:713–720.21. Yoneda K, Tomimoto A, Endo H, Iida H, Sugiyama M, Takahashi H, et al. Expression of adiponectin receptors, AdipoR1 and AdipoR2, in normal colon epithelium and colon cancer tissue. Oncol Rep. 2008. 20:479–483.22. Wang XJ, Yuan SL, Lu Q, Lu YR, Zhang J, Liu Y, et al. Potential involvement of leptin in carcinogenesis of hepatocellular carcinoma. World J Gastroenterol. 2004. 10:2478–2481.23. Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003. 278:42660–42667.24. Somasundar P, Frankenberry KA, Skinner H, Vedula G, McFadden DW, Riggs D, et al. Prostate cancer cell proliferation is influenced by leptin. J Surg Res. 2004. 118:71–82.25. Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007. 16:308–313.26. Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006. 15:1331–1335.27. Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006. 340:1158–1166.28. Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008. 101:1317–1322.29. Ribeiro R, Lopes C, Medeiros R. The link between obesity and prostate cancer: the leptin pathway and therapeutic perspectives. Prostate Cancer Prostatic Dis. 2006. 9:19–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Adiponectin Receptors and the Effects of Adiponectin and Leptin on Airway Smooth Muscle Cells
- Signaling Role of Adipocyte Leptin in Prostate Cell Proliferation Induced by Trichomonas vaginalis
- Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer
- Expression of Leptin, Leptin Receptor, Adiponectin, and Adiponectin Receptor in Ductal Carcinoma In Situ and Invasive Breast Cancer
- Correlations of Leptin, Adiponectin and Leptin/Adiponectin Ratio with Metabolic Disorders in the Childhood Obesity