Immune Netw.
2016 Jun;16(3):189-194. 10.4110/in.2016.16.3.189.
Differential Chemokine Signature between Human Preadipocytes and Adipocytes
- Affiliations
-
- 1Department of Biochemistry and Cancer Biology, Meharry Medical College, Nashville, Tennessee 37208, USA. dson@mmc.edu
- 2Department of Physiology, Meharry Medical College, Nashville, Tennessee 37208, USA.
- KMID: 2299793
- DOI: http://doi.org/10.4110/in.2016.16.3.189
Abstract
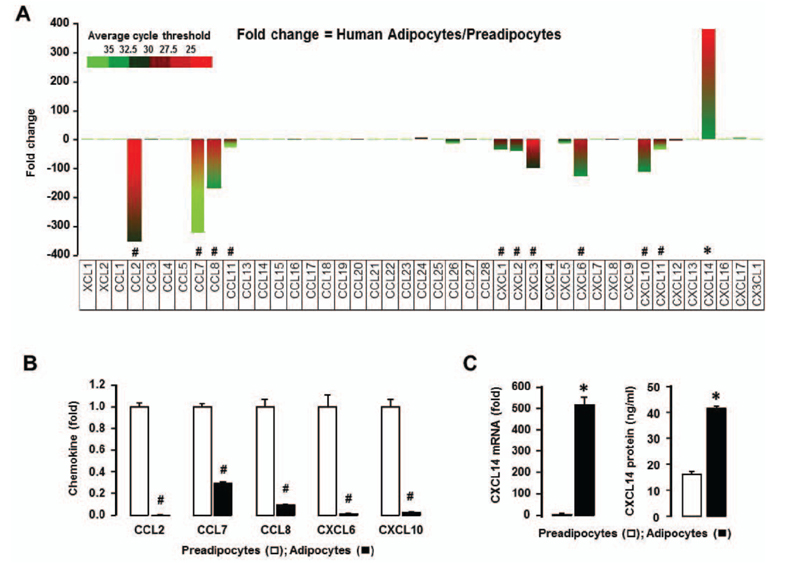
- Obesity is characterized as an accumulation of adipose tissue mass represented by chronic, low-grade inflammation. Obesity-derived inflammation involves chemokines as important regulators contributing to the pathophysiology of obesity-related diseases such as cardiovascular disease, diabetes and some cancers. The obesity-driven chemokine network is poorly understood. Here, we identified the profiles of chemokine signature between human preadipocytes and adipocytes, using PCR arrays and qRT-PCR. Both preadipocytes and adipocytes showed absent or low levels in chemokine receptors in spite of some changes. On the other hand, the chemokine levels of CCL2, CCL7-8, CCL11, CXCL1-3, CXCL6 and CXCL10-11 were dominantly expressed in preadipocytes compared to adipocytes. Interestingly, CXCL14 was the most dominant chemokine expressed in adipocytes compared to preadipocytes. Moreover, there is significantly higher protein level of CXCL14 in conditioned media from adipocytes. In addition, we analyzed the data of the chemokine signatures in adipocytes obtained from healthy lean and obese postmenopausal women based on Gene Expression Omnibus (GEO) dataset. Adipocytes from obese individuals had significantly higher levels in chemokine signature as follows: CCL2, CCL13, CCL18-19, CCL23, CCL26, CXCL1, CXCL3 and CXCL14, as compared to those from lean ones. Also, among the chemokine networks, CXCL14 appeared to be the highest levels in adipocytes from both lean and obese women. Taken together, these results identify CXCL14 as an important chemokine induced during adipogenesis, requiring further research elucidating its potential therapeutic benefits in obesity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Insulin resistance and type 2 diabetes in children
Valeria Castorani, Nella Polidori, Cosimo Giannini, Annalisa Blasetti, Francesco Chiarelli
Ann Pediatr Endocrinol Metab. 2020;25(4):217-226. doi: 10.6065/apem.2040090.045.
Reference
-
1. Wagner M, Samdal Steinskog ES, Wiig H. Adipose tissue macrophages: the inflammatory link between obesity and cancer? Expert Opin Ther Targets. 2015; 19:527–538.
Article2. Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014; 40:16–28.
Article3. Crunkhorn S. Metabolic disorders: Breaking the links between inflammation and diabetes. Nat Rev Drug Discov. 2013; 12:261.
Article4. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4:579–591.
Article5. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867.
Article6. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003; 112:1785–1788.
Article7. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010; 72:219–246.
Article8. Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der, van der, Kroesen BJ, Vonk R, Rezaee F. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011; 6:e17154.
Article9. Wang Y. Small lipid-binding proteins in regulating endothelial and vascular functions: focusing on adipocyte fatty acid binding protein and lipocalin-2. Br J Pharmacol. 2012; 165:603–621.
Article10. Chavey C, Lazennec G, Lagarrigue S, Clapé C, Iankova I, Teyssier J, Annicotte JS, Schmidt J, Mataki C, Yamamoto H, Sanches R, Guma A, Stich V, Vitkova M, Jardin-Watelet B, Renard E, Strieter R, Tuthill A, Hotamisligil GS, Vidal-Puig A, Zorzano A, Langin D, Fajas L. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009; 9:339–349.
Article11. Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006; 7:243.12. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010; 16:133–144.
Article13. Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, Sherman V, Wang HY, Phillips KJ, Webb P, Wong ST, Wang RF, Hsueh WA. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013; 17:411–422.
Article14. Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One. 2011; 6:e19541.
Article15. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
Article16. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830.
Article17. Nara N, Nakayama Y, Okamoto S, Tamura H, Kiyono M, Muraoka M, Tanaka K, Taya C, Shitara H, Ishii R, Yonekawa H, Minokoshi Y, Hara T. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem. 2007; 282:30794–30803.
Article18. Ping D, Boekhoudt GH, Rogers EM, Boss JM. Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol. 1999; 162:727–734.19. Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007; 6:1302–1312.
Article20. Kabir SM, Lee ES, Son DS. Chemokine network during adipogenesis in 3T3-L1 cells: Differential response between growth and proinflammatory factor in preadipocytes vs. adipocytes. Adipocyte. 2014; 3:97–106.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Cytotoxicity of Poly - L - lysine in Different Concentration to Preadipocytes Harvested from Living Rats
- Growth and Differentiation of Preadipocytes in Alginate and Collagen Gels
- Differentiation and Labeling of Mouse Preadipocytes for Allogenic Transplantation Study
- Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- The Role of Alginate Sponge on Growth and Differentiation of Preadipocytes