Allergy Asthma Immunol Res.
2016 Sep;8(5):461-465. 10.4168/aair.2016.8.5.461.
Soluble CD93 as a Novel Biomarker in Asthma Exacerbation
- Affiliations
-
- 1Internal Medicine Department, Medical Faculty, Kurdistan University of Medical Sciences, Sanandaj, Iran. naseh.sigari@muk.ac.ir
- 2Kurdistan Cellular & Molecular Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran.
- 3Internal Medicine Department, Kurdistan University of Medical Sciences, Sanandaj, Iran.
- 4Department of Epidemiology and Biostatistics, School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
- 5Pediatrics Department, Medical Faculty,Kurdistan University of Medical Sciences, Sanandaj, Iran.
- KMID: 2295156
- DOI: http://doi.org/10.4168/aair.2016.8.5.461
Abstract
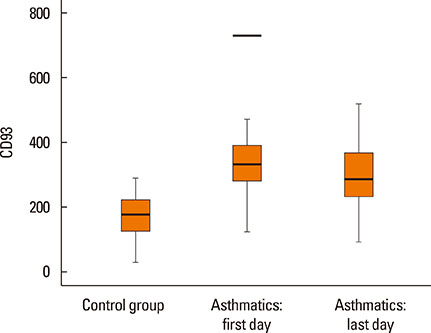
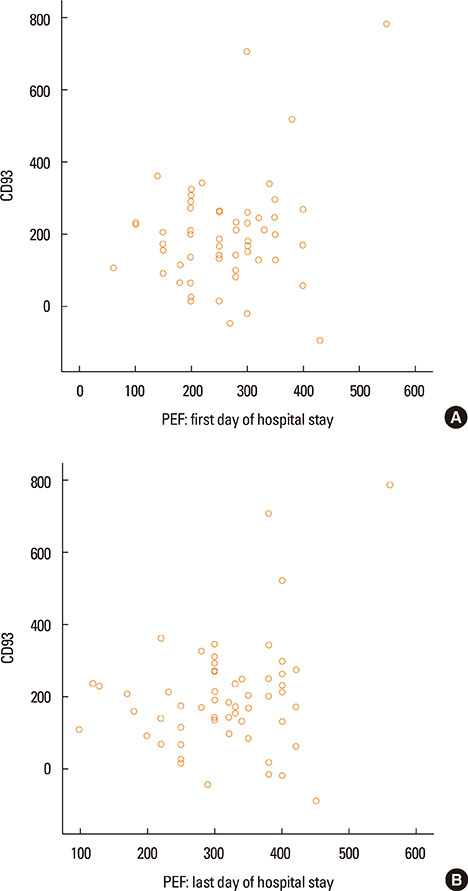
- Asthma research is shifting from studying symptoms and lung functions to the narrow-focus cellular profiles protein analysis, biomarkers, and genetic markers. The transmembrane glycoprotein CD93 is involved in endothelial cell migration, angiogenesis, leukocytes extravasation, apoptosis, innate immunity and inflammation. Relationships between the serum level of soluble CD93 (sCD93) and acute myocardial infarction/premature MI/inflammatory arthritis/skin sclerosis have recently been reported. We hypothesized that sCD93 would be elevated during the acute phase of asthma. We measured the serum level of sCD93 in 57 patients with asthma exacerbation and 57 age-and gender-matched healthy controls. Additionally, sCD93 was reassessed at the time of discharge from the hospital. Clinical characteristics and peak expiratory flow (PEF) of the patients were assessed. The primary outcome was the comparison of serum level of sCD93 between asthmatics and healthy subjects. The sCD93 values ranged from 128 to 789 ng/mL in asthmatics (345.83±115.81) and from 31 to 289 ng/mL in control subjects (169.46±62.43). The difference between the 2 groups was statistically significant (P<0.001). The association between sCD93 and asthma remained significant after adjusting for age, sex, and BMI. The differences between asthmatics and controls remained significant on the last day of hospital stay. The association between sCD93 and PEF was not significant. In conclusion, the serum level of soluble CD93 is increased in patients with asthma exacerbation. It also showed that serum levels of sCD93 decreased with treatment of asthma attack. The clinical usefulness of determination of sCD93 as a biomarker of asthma requires further studies.
MeSH Terms
Figure
Cited by 1 articles
-
Soluble CD93 in Serum as a Marker of Allergic Inflammation
Hye Jung Park, Heejae Han, Sang Chul Lee, Young Woong Son, Da Woon Sim, Kyung Hee Park, Yoon Hee Park, Kyoung Yong Jeong, Jung-Won Park, Jae-Hyun Lee
Yonsei Med J. 2017;58(3):598-603. doi: 10.3349/ymj.2017.58.3.598.
Reference
-
1. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention [Internet]. place unknown: Global Initiative for Asthma (GINA);2015. cited 2016 Jan. Available from: http://www.ginasthma.org/documents/4.2. National Institutes of Health, U.S. Department of Health and Human Services. National Heart, Lung, and Blood Institute (US). Guidelines for the diagnosis and management of asthma (EPR-3) [Internet]. National Heart, Lung, and Blood Institute;2007. cited 2016 Jan. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines.3. Zhao CN, Fan Y, Huang JJ, Zhang HX, Gao T, Wang C, et al. The Association of GSDMB and ORMDL3 gene polymorphisms with asthma: a meta-analysis. Allergy Asthma Immunol Res. 2015; 7:175–185.4. Sircar G, Saha B, Bhattacharya SG, Saha S. Allergic asthma biomarkers using systems approaches. Front Genet. 2014; 4:308.5. Jeon JW, Jung JG, Shin EC, Choi HI, Kim HY, Cho ML, et al. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J Immunol. 2010; 185:4921–4927.6. Liu C, Cui Z, Wang S, Zhang D. CD93 and GIPC expression and localization during central nervous system inflammation. Neural Regen Res. 2014; 9:1995–2001.7. Harhausen D, Prinz V, Ziegler G, Gertz K, Endres M, Lehrach H, et al. CD93/AA4.1: a novel regulator of inflammation in murine focal cerebral ischemia. J Immunol. 2010; 184:6407–6417.8. Chevrier S, Genton C, Kallies A, Karnowski A, Otten LA, Malissen B, et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc Natl Acad Sci U S A. 2009; 106:3895–3900.9. Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997; 6:119–129.10. McGreal EP, Ikewaki N, Akatsu H, Morgan BP, Gasque P. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. J Immunol. 2002; 168:5222–5232.11. Steinberger P, Szekeres A, Wille S, Stöckl J, Selenko N, Prager E, et al. Identification of human CD93 as the phagocytic C1q receptor (C1qRp) by expression cloning. J Leukoc Biol. 2002; 71:133–140.12. Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, Taylor PR, Bygrave AE, Thompson RD, et al. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J Immunol. 2004; 172:3406–3414.13. Jakel A, Qaseem AS, Kishore U, Sim RB. Ligands and receptors of lung surfactant proteins SP-A and SP-D. Front Biosci (Landmark Ed). 2013; 18:1129–1140.14. Greenlee MC, Sullivan SA, Bohlson SS. Detection and characterization of soluble CD93 released during inflammation. Inflamm Res. 2009; 58:909–919.15. Ikewaki N, Tamauchi H, Yamada A, Mori N, Yamao H, Inoue H, et al. A unique monoclonal antibody mNI-11 rapidly enhances spread formation in human umbilical vein endothelial cells. J Clin Immunol. 2000; 20:317–324.16. Park M, Tenner AJ. Cell surface expression of C1qRP/CD93 is stabilized by O-glycosylation. J Cell Physiol. 2003; 196:512–522.17. Bohlson SS, Silva R, Fonseca MI, Tenner AJ. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol. 2005; 175:1239–1247.18. Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013; 5:343–347.19. Sigari N, Ghasri H. Correlation between hs-CRP and asthma control indices. Tanaffos. 2013; 12:44–48.20. Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland-van der Zee AH, Koenderman L. Clinical utility of asthma biomarkers: from bench to bedside. Biologics. 2013; 7:199–210.21. Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011; 242:220–232.22. Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003; 5:1317–1327.23. Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009; 155:559–566.24. Greenlee-Wacker MC, Briseño C, Galvan M, Moriel G, Velázquez P, Bohlson SS. Membrane-associated CD93 regulates leukocyte migration and C1q-hemolytic activity during murine peritonitis. J Immunol. 2011; 187:3353–3361.25. Mälarstig A, Silveira A, Wågsäter D, Öhrvik J, Bäcklund A, Samnegård A, et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J Intern Med. 2011; 270:229–236.26. Youn JC, Yu HT, Jeon JW, Lee HS, Jang Y, Park YW, et al. Soluble CD93 levels in patients with acute myocardial infarction and its implication on clinical outcome. PLoS One. 2014; 9:e96538.27. Yanaba K, Asano Y, Noda S, Akamata K, Aozasa N, Taniguchi T, et al. Augmented production of soluble CD93 in patients with systemic sclerosis and clinical association with severity of skin sclerosis. Br J Dermatol. 2012; 167:542–547.28. Raedler D, Ballenberger N, Klucker E, Böck A, Otto R, Prazeres da Costa O, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015; 135:81–91.29. Zasłona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz-Tennenbaum S, Osterholzer JJ, et al. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol. 2014; 193:4245–4253.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Soluble CD93 in Serum as a Marker of Allergic Inflammation
- Effect of air pollution on acute exacerbation of adult asthma in Seoul, Korea
- The Effects of Bronchiectasis on Asthma Exacerbation
- Seasonality of asthma exacerbation in children caused by respiratory virus infection and allergen sensitization
- Clinical significance of serum vascular endothelial growth factor in young male asthma patients