J Breast Cancer.
2012 Sep;15(3):306-312. 10.4048/jbc.2012.15.3.306.
Impact of Breast Cancer and Combination Chemotherapy on Oxidative Stress, Hepatic and Cardiac Markers
- Affiliations
-
- 1Biochemistry Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt. kaamin10@yahoo.com
- 2Chemistry Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt.
- 3Clinical Oncology Department, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt.
- KMID: 2286456
- DOI: http://doi.org/10.4048/jbc.2012.15.3.306
Abstract
- PURPOSE
Carcinoma of the breast is the most prevalent cancer among Egyptian women and constitutes 29% of National Cancer Institute cases. The aim of this study was to determine the effect of breast cancer on oxidative stress, cardiac markers and liver function tests, moreover the role of 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC) in the treatment of breast cancer and its mechanism through changing the measured markers.
METHODS
Forty female breast cancer patients who were admitted to the Department of Oncology of the Beni-Suef University Hospital were enrolled in the study. This study included three arms: a control group of healthy age-matched females (n=20), breast cancer patients who weren't receiving treatment (n=20), and patients undergoing treatment with anticancer combination drugs FAC (n=20). Blood samples collected from the control subjects and patients were analysed to determine levels of catalase, reduced glutathione (GSH), uric acid, nitric oxide (NO), malondialdehyde, creatine kinase (CK), lactate dehydrogenase (LDH), liver enzymes (alanine aminotransferase and aspartate aminotransferase), and creatinine.
RESULTS
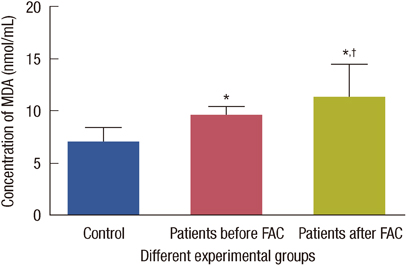
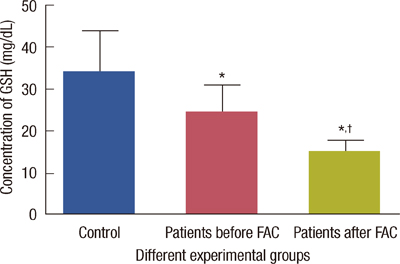
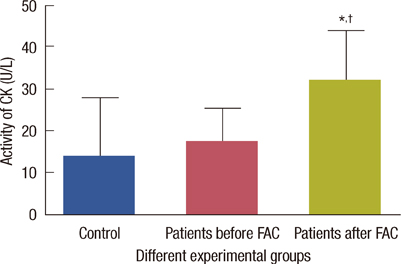
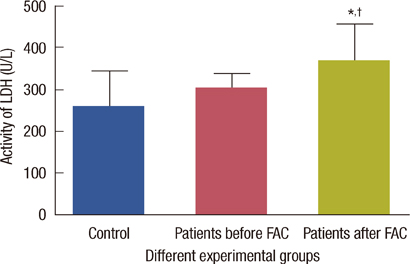
The levels of catalase and GSH were significantly reduced (p<0.05) in breast carcinoma and FAC treated breast cancer patients. The lipid peroxidation and NO levels were significantly enhanced in both untreated and FAC treated breast cancer patients. The CK and LDH were significantly enhanced (p<0.05) in the FAC group.
CONCLUSION
The results from the present study show that oxidative stress is implicated in breast carcinoma and chemotherapy aggravates this oxidative stress which causes damage to many cellular targets and has the main side effect of cardiotoxicity.
MeSH Terms
-
Aspartic Acid
Breast
Breast Neoplasms
Catalase
Creatine Kinase
Cyclophosphamide
Doxorubicin
Drug Therapy, Combination
Female
Fluorouracil
Glutathione
Humans
L-Lactate Dehydrogenase
Lipid Peroxidation
Liver
Liver Function Tests
Malondialdehyde
National Cancer Institute (U.S.)
Nitric Oxide
Oxidative Stress
Uric Acid
Aspartic Acid
Catalase
Creatine Kinase
Cyclophosphamide
Doxorubicin
Fluorouracil
Glutathione
L-Lactate Dehydrogenase
Malondialdehyde
Nitric Oxide
Uric Acid
Figure
Reference
-
1. Polyak K. On the birth of breast cancer. Biochim Biophys Acta. 2001. 1552:1–13.
Article2. Gönenç A, Erten D, Aslan S, Akinci M, Simşek B, Torun M. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int. 2006. 30:376–380.
Article3. Yeh CC, Hou MF, Tsai SM, Lin SK, Hsiao JK, Huang JC, et al. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clin Chim Acta. 2005. 361:104–111.
Article4. Kang DH. Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues. 2002. 13:540–549.
Article5. Birnboim HC. DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Science. 1982. 215:1247–1249.
Article6. Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000. 32:157–170.
Article7. Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992. 19:670–686.8. Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004. 3:294–300.
Article9. Simůnek T, Stérba M, Popelová O, Adamcová M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009. 61:154–171.
Article10. Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J. 2008. 49:640–643.11. Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970. 34:30–38.
Article12. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963. 61:882–888.13. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001. 5:62–71.
Article14. Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978. 90:37–43.
Article15. Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. 1998. 3rd ed. Philadelphia: W.B. Saunders Company;1034.16. Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980. 26:227–231.
Article17. Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010. 3:23–34.
Article18. Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, et al. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003. 523-524:163–172.
Article19. Prabasheela B, Singh AK, Fathima A, Pragulbh K, Deka NJ, Kumar R. Association between antioxidant enzymes and breast cancer. Recent Res Sci Technol. 2011. 3:93–95.20. Kasapović J, Pejić S, Stojiljković V, Todorović A, Radošević-Jelić L, Saičić ZS, et al. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and cyclophosphamide. Clin Biochem. 2010. 43:1287–1293.
Article21. Switzer CH, Glynn SA, Ridnour LA, Cheng RY, Vitek MP, Ambs S, et al. Nitric oxide and protein phosphatase 2A provide novel therapeutic opportunities in ER-negative breast cancer. Trends Pharmacol Sci. 2011. 32:644–651.
Article22. Ambs S, Glynn SA. Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle. 2011. 10:619–624.
Article23. Alshabanah OA, Hafez MM, Al-Harbi MM, Hassan ZK, Al Rejaie SS, Asiri YA, et al. Doxorubicin toxicity can be ameliorated during antioxidant L-carnitine supplementation. Oxid Med Cell Longev. 2010. 3:428–433.
Article24. Nohl H. Demonstration of the existence of an organo-specific NADH dehydrogenase in heart mitochondria. Eur J Biochem. 1987. 169:585–591.
Article25. Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982. 257:5751–5754.
Article26. Kaithwas G, Dubey K, Pillai KK. Effect of aloe vera (Aloe barbadensis Miller) gel on doxorubicin-induced myocardial oxidative stress and calcium overload in albino rats. Indian J Exp Biol. 2011. 49:260–268.27. Koti BC, Vishwanathswamy AH, Wagawade J, Thippeswamy AH. Cardioprotective effect of lipistat against doxorubicin induced myocardial toxicity in albino rats. Indian J Exp Biol. 2009. 47:41–46.28. Goormaghtigh E, Huart P, Praet M, Brasseur R, Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem. 1990. 35:247–257.29. Deng S, Kulle B, Hosseini M, Schlüter G, Hasenfuss G, Wojnowski L, et al. Dystrophin-deficiency increases the susceptibility to doxorubicin-induced cardiotoxicity. Eur J Heart Fail. 2007. 9:986–994.
Article30. Nohl H, Gille L, Staniek K. The exogenous NADH dehydrogenase of heart mitochondria is the key enzyme responsible for selective cardiotoxicity of anthracyclines. Z Naturforsch C. 1998. 53:279–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced Radiosensitivity and Chemosensitivity of Breast Cancer Cells by 2-Deoxy-D-Glucose in Combination Therapy
- Mono- and Combination Chemotherapy for Metastatic Breast Cancer: An Increamental Step Forward
- Chemotherapy in Breast Cancer
- The Effect of Neoadjuvant Chemotherapy on the Biological Prognostic Markers in Breast Cancer Patients
- Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review