Korean J Physiol Pharmacol.
2015 Jul;19(4):319-325. 10.4196/kjpp.2015.19.4.319.
Polymorphisms of SLC22A9 (hOAT7) in Korean Females with Osteoporosis
- Affiliations
-
- 1Department of Tropical Medicine and Parasitology, College of Medicine, Inha University, Incheon 400-712, Korea. shcha@inha.ac.kr
- 2Department of Physiology and Biophysics, College of Medicine, Inha University, Incheon 400-712, Korea.
- KMID: 2285574
- DOI: http://doi.org/10.4196/kjpp.2015.19.4.319
Abstract
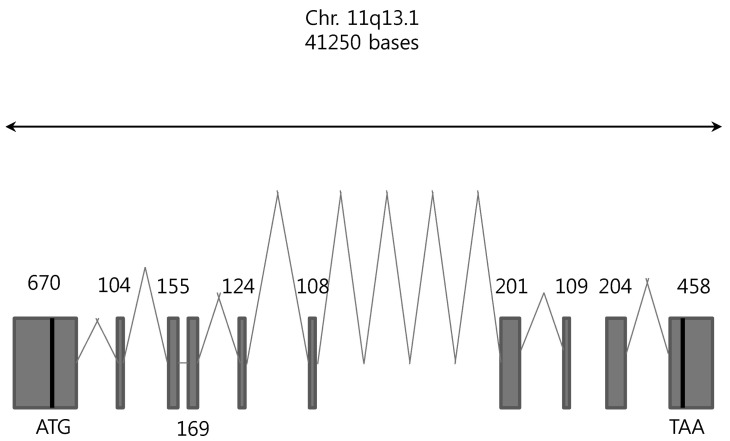
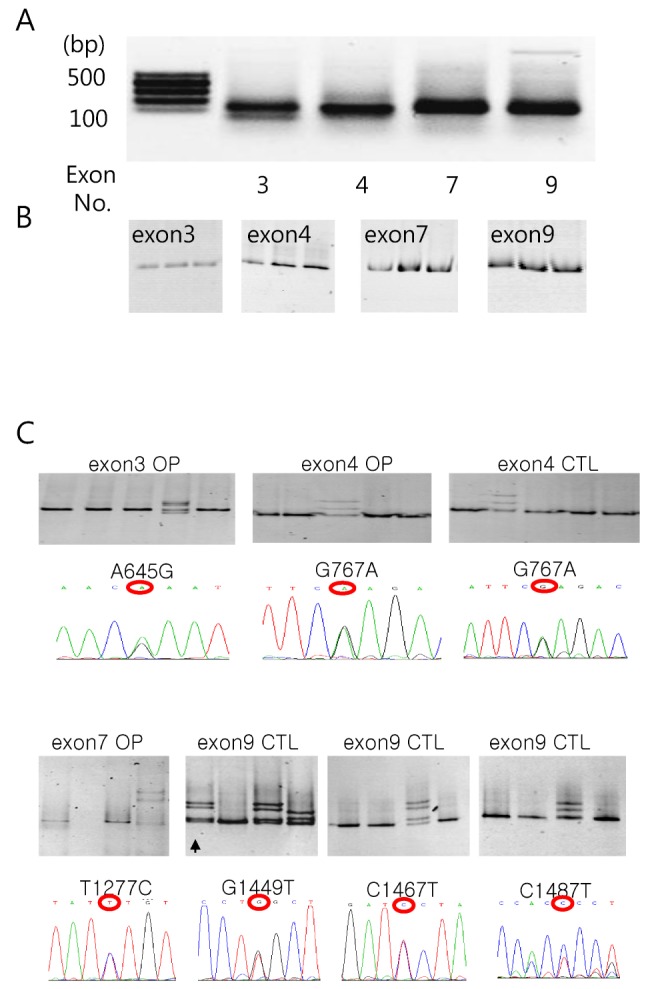
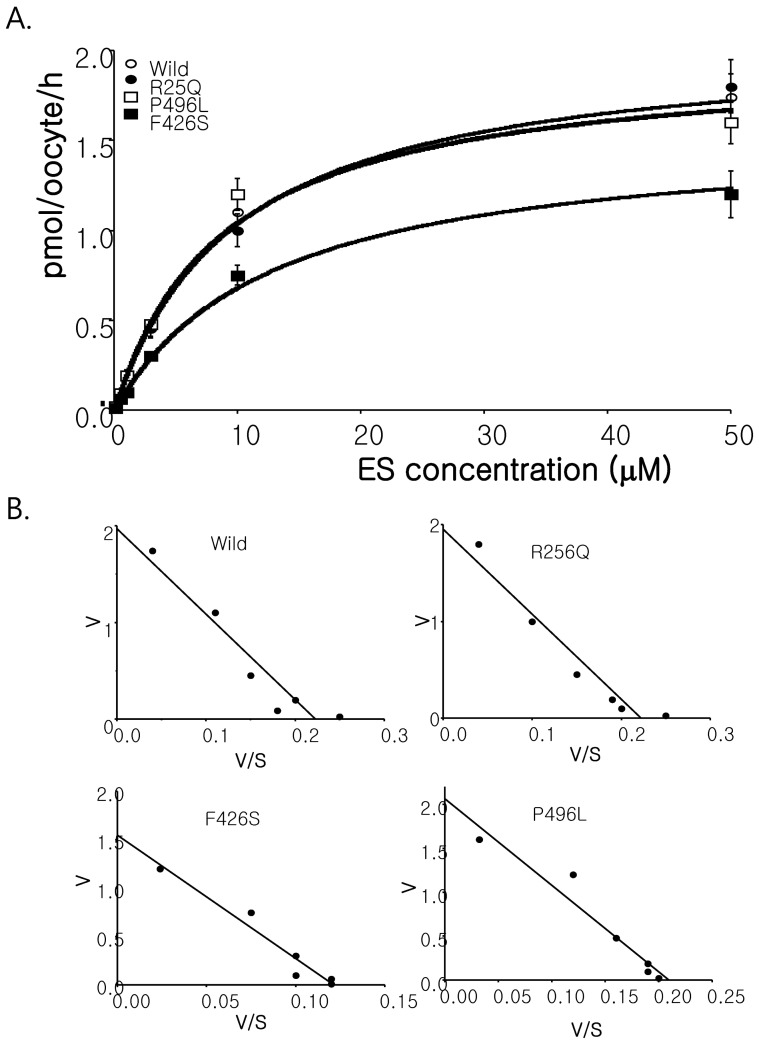
- Among solute carrier proteins, the organic anion transporters (OATs) play an important role for the elimination or reabsorption of endogenous and exogenous negatively charged anionic compounds. Among OATs, SLC22A9 (hOAT7) transports estrone sulfate with high affinity. The net decrease of estrogen, especially in post-menopausal women induces rapid bone loss. The present study was performed to search the SNP within exon regions of SLC22A9 in Korean females with osteoporosis. Fifty healthy controls and 50 osteoporosis patients were screened for the genetic polymorphism in the coding region of SLC22A9 using GC-clamped PCR and denaturing gradient gel electrophoresis (DGGE). Six SNPs were found on the SLC22A9 gene from Korean women with/without osteoporosis. The SNPs were located as follows: two SNPs in the osteoporosis group (A645G and T1277C), three SNPs in the control group (G1449T, C1467T and C1487T) and one SNP in both the osteoporosis and control groups (G767A). The G767A, T1277C and C1487T SNPs result in an amino acid substitution, from synonymous vs nonsynonymous substitution arginine to glutamine (R256Q), phenylalanine to serine (F426S) and proline to leucine (P496L), respectively. The Km values and Vmax of the wild type, R256Q, P496L and F426S were 8.84, 8.87, 9.83 and 12.74 microM, and 1.97, 1.96, 2.06 and 1.55 pmol/oocyte/h, respectively. The present study demonstrates that the SLC22A9 variant F426S is causing inter-individual variation that is leading to the differences in transport of the steroid sulfate conjugate (estrone sulfate) and, therefore this could be used as a marker for certain disease including osteoporosis.
Keyword
MeSH Terms
-
Amino Acid Substitution
Arginine
Avena
Carrier Proteins
Clinical Coding
Denaturing Gradient Gel Electrophoresis
Estrogens
Estrone
Exons
Female
Glutamine
Humans
Leucine
Organic Anion Transporters
Osteoporosis*
Phenylalanine
Polymerase Chain Reaction
Polymorphism, Genetic
Polymorphism, Single Nucleotide
Proline
Serine
Arginine
Carrier Proteins
Estrogens
Estrone
Glutamine
Leucine
Organic Anion Transporters
Phenylalanine
Proline
Serine
Figure
Reference
-
1. Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002; 23:279–302. PMID: 12050121.
Article2. Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass: facts and uncertainties. Arch Pediatr. 1995; 2:460–468. PMID: 7640740.3. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342. PMID: 12748652.
Article4. Wang FS, Ko JY, Lin CL, Wu HL, Ke HJ, Tai PJ. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone. 2007; 40:485–492. PMID: 17055793.
Article5. Muir M, Romalo G, Wolf L, Elger W, Schweikert HU. Estrone sulfate is a major source of local estrogen formation in human bone. J Clin Endocrinol Metab. 2004; 89:4685–4692. PMID: 15356081.
Article6. Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest. 1997; 100:1755–1759. PMID: 9312174.
Article7. Khosla S, Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274. PMID: 9661593.
Article9. Hui SL, Perkins AJ, Zhou L, Longcope C, Econs MJ, Peacock M, McClintock C, Johnston CC Jr. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002; 87:1539–1543. PMID: 11932278.
Article10. Noel CT, Reed MJ, Jacobs HS, James VH. The plasma concentration of oestrone sulphate in postmenopausal women: lack of diurnal variation, effect of ovariectomy, age and weight. J Steroid Biochem. 1981; 14:1101–1105. PMID: 7198170.
Article11. Roberts KD, Rochefort JG, Bleau G, Chapdelaine A. Plasma estrone sulfate levels in postmenopausal women. Steroids. 1980; 35:179–187. PMID: 7376217.
Article12. Hawkins RA, Oakey RE. Estimation of oestrone sulphate, oestradiol-17beta and oestrone in peripheral plasma: concentrations during the menstrual cycle and in men. J Endocrinol. 1974; 60:3–17. PMID: 4812465.13. Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001; 59:1277–1286. PMID: 11306713.
Article14. Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, Kanai Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007; 45:1046–1055. PMID: 17393504.
Article15. Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000; 275:4507–4512. PMID: 10660625.
Article16. Deighton CM, Walker DJ, Griffiths ID, Roberts DF. The contribution of HLA to rheumatoid arthritis. Clin Genet. 1989; 36:178–182. PMID: 2676268.17. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003; 423:356–361. PMID: 12748655.
Article18. Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: Genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol. 2006; 2:425–433. PMID: 16932734.
Article19. Masi AT, Aldag JC, Chatterton RT. Sex hormones and risks of rheumatoid arthritis and developmental or environmental influences. Ann N Y Acad Sci. 2006; 1069:223–235. PMID: 16855149.
Article20. Lee WK, Kwak JO, Hwang JS, Suh CK, Cha SH. Identification and characterization of single nucleotide polymorphisms of SLC22A11 (hOAT4) in Korean women osteoporosis patients. Mol Cells. 2008; 25:265–271. PMID: 18414001.21. Mumm S, Jones J, Finnegan P, Henthorn PS, Podgornik MN, Whyte MP. Denaturing gradient gel electrophoresis analysis of the tissue nonspecific alkaline phosphatase isoenzyme gene in hypophosphatasia. Mol Genet Metab. 2002; 75:143–153. PMID: 11855933.
Article22. Wu Y, Hayes VM, Osinga J, Mulder IM, Looman MW, Buys CH, Hofstra RM. Improvement of fragment and primer selection for mutation detection by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1998; 26:5432–5440. PMID: 9826769.
Article23. Van Orsouw NJ, Vijg J. Design and application of 2-D DGGE-based gene mutational scanning tests. Genet Anal. 1999; 14:205–213. PMID: 10084116.
Article24. Kwak JO, Kim HW, Oh KJ, Kim DS, Han KO, Cha SH. Co-localization and interaction of organic anion transporter 1 with caveolin-2 in rat kidney. Exp Mol Med. 2005; 37:204–212. PMID: 16000875.
Article25. Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999; 84:4677–4694. PMID: 10599737.
Article26. Bilezikian JP. Sex steroids, mice, and men: when androgens and estrogens get very close to each other. J Bone Miner Res. 2002; 17:563–566. PMID: 11918214.
Article27. Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R1357–R1365. PMID: 16322349.
Article28. Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 2007; 21:3592–3605. PMID: 17595345.
Article29. Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003; 371:897–905. PMID: 12568656.
Article30. Patel P, Weerasekera N, Hitchins M, Boyd CA, Johnston DG, Williamson C. Semi quantitative expression analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C,OATP-D, OATP-E and NTCP gene transcripts in 1st and 3rd trimester human placenta. Placenta. 2003; 24:39–44. PMID: 12495658.31. Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab. 2003; 284:E390–E398. PMID: 12409283.32. Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003; 83:633–671. PMID: 12663868.33. Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007; 35:595–601. PMID: 17237156.
Article34. Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006; 291:E1295–E1304. PMID: 17082346.
Article35. Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005; 33:434–439. PMID: 15608127.
Article36. Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004; 279:7213–7222. PMID: 14660639.
Article37. Conrad S, Kauffmann HM, Ito K, Deeley RG, Cole SP, Schrenk D. Identification of human multidrug resistance protein 1 (MRP1) mutations and characterization of a G671V substitution. J Hum Genet. 2001; 46:656–663. PMID: 11721885.
Article38. Lee SS, Jeong HE, Yi JM, Jung HJ, Jang JE, Kim EY, Lee SJ, Shin JG. Identification and functional assessment of BCRP polymorphisms in a Korean population. Drug Metab Dispos. 2007; 35:623–632. PMID: 17237154.39. Keitel V, Nies AT, Brom M, Hummel-Eisenbeiss J, Spring H, Keppler D. A common Dubin-Johnson syndrome mutation impairs protein maturation and transport activity of MRP2 (ABCC2). Am J Physiol Gastrointest Liver Physiol. 2003; 284:G165–G174. PMID: 12388192.40. Jamroziak K, Balcerczak E, Smolewski P, Robey RW, Cebula B, Panczyk M, Kowalczyk M, Szmigielska-KapłZon A, Mirowski M, Bates SE, Robak T. MDR1 (ABCB1) gene polymorphism C3435T is associated with P-glycoprotein activity in B-cell chronic lymphocytic leukemia. Pharmacol Rep. 2006; 58:720–728. PMID: 17085864.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of LRP5 gene polymorphisms with bone mineral density and bone responsiveness to hormone therapy in postmenopausal Korean women
- Pharmacogenetics of Response to Bisphosphonate Treatment in Postmenopausal Osteoporosis: A Prospective Study
- Severe Osteoporosis and Advanced Severe Osteoporosis
- Effect of Postoperative Parathyroid Hormone Administration on Osteoporotic Intertrochanteric Fractures of Females
- Issues of Compliance in Osteoporosis Medication