Korean J Physiol Pharmacol.
2015 Mar;19(2):105-109. 10.4196/kjpp.2015.19.2.105.
NgR1 Expressed in P19 Embryonal Carcinoma Cells Differentiated by Retinoic Acid Can Activate STAT3
- Affiliations
-
- 1College of Pharmacy, Korea University, Sejong 339-700, Korea. spark123@korea.ac.kr
- 2Bioevaluation Center, KRIBB, Ochang 363-883, Korea.
- 3College of Pharmacy, Chungbuk National University, Cheongju 362-763, Korea.
- 4College of Pharmacy, Chungnam National University, Daejeon 305-764, Korea.
- 5Research Driven Hospital, Korea University Guro Hospital, Biomedical Research Center, Seoul 152-703, Korea.
- KMID: 2285561
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.105
Abstract
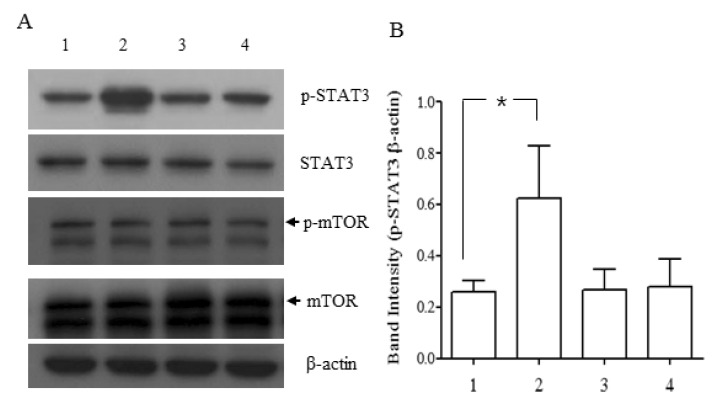
- NgR1, a Nogo receptor, is involved in inhibition of neurite outgrowth and axonal regeneration and regulation of synaptic plasticity. P19 embryonal carcinoma cells were induced to differentiate into neuron-like cells using all trans-retinoic acid and the presence and/or function of cellular molecules, such as NgR1, NMDA receptors and STAT3, were examined. Neuronally differentiated P19 cells expressed the mRNA and protein of NgR1, which could stimulate the phosphorylation of STAT3 when activated by Nogo-P4 peptide, an active segment of Nogo-66. During the whole period of differentiation, mRNAs of all of the NMDA receptor subtypes tested (NR1, NR2A-2D) were consistently expressed, which meant that neuronally differentiated P19 cells maintained some characteristics of neurons, especially central nervous system neurons. Our results suggests that neuronally differentiated P19 cells expressing NgR1 may be an efficient and convenient in vitro model for studying the molecular mechanism of cellular events that involve NgR1 and its binding partners, and for screening compounds that activate or inhibit NgR1.
Keyword
MeSH Terms
Figure
Reference
-
1. McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. 1993; 37:135–140. PMID: 8507558.2. Canzoniero LM, Sensi SL, Turetsky DM, Finley MF, Choi DW, Huettner JE. Glutamate receptor-mediated calcium entry in neurons derived from P19 embryonal carcinoma cells. J Neurosci Res. 1996; 45:226–236. PMID: 8841983.
Article3. Grant ER, Errico MA, Emanuel SL, Benjamin D, McMillian MK, Wadsworth SA, Zivin RA, Zhong Z. Protection against glutamate toxicity through inhibition of the p44/42 mitogen-activated protein kinase pathway in neuronally differentiated P19 cells. Biochem Pharmacol. 2001; 62:283–296. PMID: 11434901.4. Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010; 11:799–811. PMID: 21045861.
Article5. Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002; 22:5505–5515. PMID: 12097502.
Article6. Liu YY, Jin WL, Liu HL, Ju G. Electron microscopic localization of Nogo-A at the postsynaptic active zone of the rat. Neurosci Lett. 2003; 346:153–156. PMID: 12853107.
Article7. Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008; 28:2753–2765. PMID: 18337405.
Article8. Takei Y. Phosphorylation of Nogo receptors suppresses Nogo signaling, allowing neurite regeneration. Sci Signal. 2009; 2:ra14. PMID: 19336839.
Article9. Gao Y, Wang B, Xiao Z, Chen B, Han J, Wang X, Zhang J, Gao S, Zhao Y, Dai J. Nogo-66 regulates nanog expression through stat3 pathway in murine embryonic stem cells. Stem Cells Dev. 2010; 19:53–60. PMID: 19400741.
Article10. Wang B, Xiao Z, Chen B, Han J, Gao Y, Zhang J, Zhao W, Wang X, Dai J. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS One. 2008; 3:e1856. PMID: 18365011.
Article11. Machida Y, Murai K, Miyake K, Iijima S. Expression of chromatin remodeling factors during neural differentiation. J Biochem. 2001; 129:43–49. PMID: 11134956.
Article12. Barrette B, Vallières N, Dubé M, Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 2007; 34:519–538. PMID: 17234430.
Article13. Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000; 403:434–439. PMID: 10667796.
Article14. GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000; 403:439–444. PMID: 10667797.
Article15. Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000; 403:383–384. PMID: 10667780.
Article16. Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001; 409:341–346. PMID: 11201742.
Article17. Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002; 417:941–944. PMID: 12068310.
Article18. Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002; 35:283–290. PMID: 12160746.
Article19. Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005; 25:808–822. PMID: 15673660.
Article20. Ulrich H, Majumder P. Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: from basic research towards clinical applications. Cell Prolif. 2006; 39:281–300. PMID: 16872363.
Article21. Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med. 2009; 361:302–303. PMID: 19605837.
Article22. Hur SW, Park JM. Long-term potentiation of excitatory synaptic strength in spinothalamic tract neurons of the rat spinal cord. Korean J Physiol Pharmacol. 2013; 17:553–558. PMID: 24381506.
Article23. Peng X, Kim J, Zhou Z, Fink DJ, Mata M. Neuronal Nogo-A regulates glutamate receptor subunit expression in hippocampal neurons. J Neurochem. 2011; 119:1183–1193. PMID: 21985178.
Article24. Kulikov AV, Rzhaninova AA, Goldshtein DV, Boldyrev AA. Expression of NMDA receptors in multipotent stromal cells of human adipose tissue under conditions of retinoic acid-induced differentiation. Bull Exp Biol Med. 2007; 144:626–629. PMID: 18642726.
Article25. Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002; 22:6570–6577. PMID: 12151536.
Article26. Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003; 23:1416–1423. PMID: 12598630.
Article27. McDonald CL, Bandtlow C, Reindl M. Targeting the Nogo receptor complex in diseases of the central nervous system. Curr Med Chem. 2011; 18:234–244. PMID: 21110803.28. Willi R, Schwab ME. Nogo and Nogo receptor: relevance to schizophrenia? Neurobiol Dis. 2013; 54:150–157. PMID: 23369871.
Article29. Lee JY, Petratos S. Multiple sclerosis: does Nogo play a role? Neuroscientist. 2013; 19:394–408. PMID: 23423307.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuronal Differentiatin of P19 Cells
- Induction of Embryonal Teratocarcinoma Cell(P19 cell) to Differentiate into Smooth Muscle Cell
- Enhanced Cardiomyogenic Differentiation of P19 Embryonal Carcinoma Stem Cells
- Role of Cardiac Transcription Factor Nkx2.5 on Cardiomyoplasty Model in vitro
- Bis Is Involved in Glial Differentiation of P19 Cells Induced by Retinoic Acid