Korean Circ J.
2009 May;39(5):198-204. 10.4070/kcj.2009.39.5.198.
Enhanced Cardiomyogenic Differentiation of P19 Embryonal Carcinoma Stem Cells
- Affiliations
-
- 1Department of Cardiology, College of Medicine, Korea University, Seoul, Korea. dslmd@kumc.or.kr
- KMID: 1826019
- DOI: http://doi.org/10.4070/kcj.2009.39.5.198
Abstract
-
BACKGROUND AND OBJECTIVES: We investigated the effects of different concentrations of serum, 5-azacytidine, and culture time on the cardiomyogenic differentiation of P19 embryonal carcinoma stem cells in the course of developing an efficient protocol for generating the cardiomyogenic lineage.
MATERIALS AND METHODS
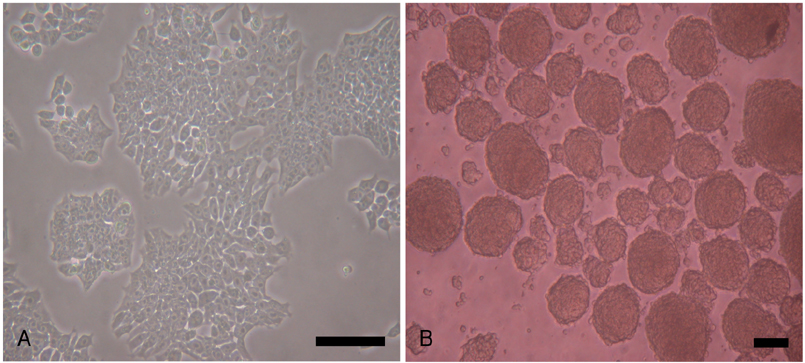
P19 cells were plated at a density of 1x10(6) cells on 10-cm bacterial dishes for 96 hours in the presence of 1% dimethyl sulfoxide to form embryoid bodies. The embryoid bodies were cultured in medium with 2% or 10% fetal bovine serum for an additional 10 or 15 consecutive days in the presence of 0, 1, or 3 microM 5-azacytidine.
RESULTS
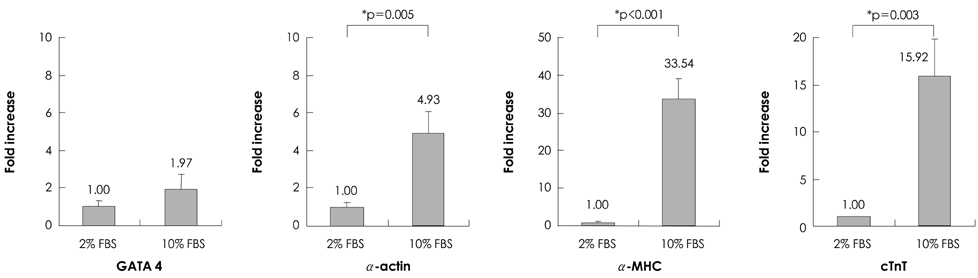
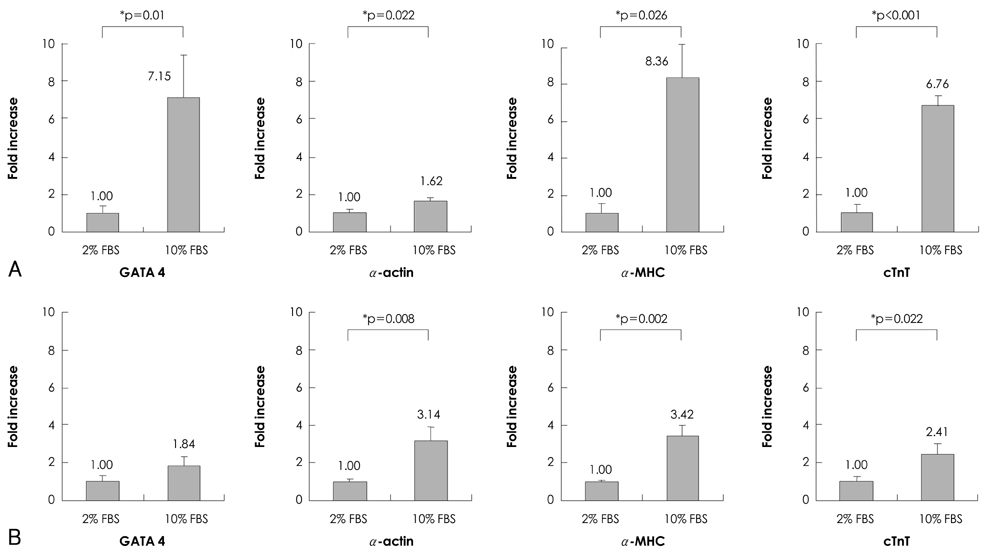
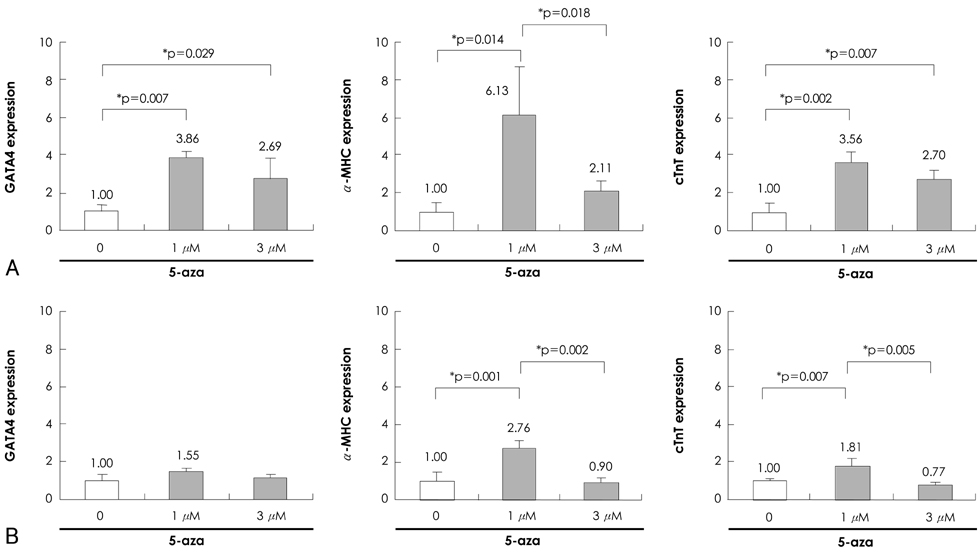
Quantitative real-time polymerase chain reaction (PCR) analysis showed that the messenger ribonucleic acid (mRNA) expression of cardiac muscle-specific genes, such as GATA4, alpha-actin, alpha-myosin heavy chain, and cardiac troponin T, were significantly higher in the 15-day culture groups than in the 10-day culture groups. Furthermore, the cardiac muscle-specific genes were expressed more in the high-serum groups compared to the low-serum groups regardless of the culture time. Cardiomyogenic differentiation of the P19 cells was most effective in 1 microM 5-azacytidine regardless of the serum concentrations. In addition, the stimulation effects of 5-azacytidine on cardiomyogenic differentiation were more significant under low-serum culture conditions compared to high-serum culture conditions. Cardiomyogenic differentiation of P19 cells was further confirmed by immunostaining with cardiac muscle-specific antibodies.
CONCLUSION
Taken together, these results demonstrated that cardiomyogenic differentiation of P19 cells was enhanced by a combination of different experimental factors.
MeSH Terms
-
Actins
Antibodies
Azacitidine
Carcinoma, Embryonal
Cell Differentiation
Dimethyl Sulfoxide
Embryoid Bodies
Embryonal Carcinoma Stem Cells
Myocytes, Cardiac
Real-Time Polymerase Chain Reaction
RNA
Safrole
Troponin T
Ventricular Myosins
Actins
Antibodies
Azacitidine
Dimethyl Sulfoxide
RNA
Safrole
Troponin T
Ventricular Myosins
Figure
Reference
-
1. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.2. Kajstura J, Rota M, Whang B, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005. 96:127–137.3. Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007. 104:17783–17788.4. Kim YS, Ahn Y, Hong MH, et al. Therapeutic potential of umbilical cord blood-derived mesenchymal stem cells in ischemic myocardium. Korean Circ J. 2008. 38:446–454.5. Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004. 109:1543–1549.6. Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004. 10:494–501.7. McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982. 299:165–167.8. van der Heyden MA, Defize LH. Twenty one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovasc Res. 2003. 58:292–302.9. Choi SC, Yoon J, Shim WJ, Ro YM, Lim DS. 5-azacytidine induces cardiac differentiation of P19 embryonic stem cells. Exp Mol Med. 2004. 36:515–523.10. McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. 1993. 37:135–140.11. Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999. 103:697–705.12. Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002. 91:501–508.13. Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003. 100:12313–12318.14. Bettiol E, Sartiani L, Chicha L, Krause KH, Cerbai E, Jaconi ME. Fetal bovine serum enables cardiac differentiation of human embryonic stem cells. Differentiation. 2007. 75:669–681.15. Taha MF, Valojerdi MR. Effect of bone morphogenetic protein-4 on cardiac differentiation from mouse embryonic stem cells in serum-free and low-serum media. Int J Cardiol. 2008. 127:78–87.16. Passier R, Oostwaard DW, Snapper J, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005. 23:772–780.17. Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells: the role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007. 6:593–597.18. Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003. 75:775–779.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuronal Differentiatin of P19 Cells
- Role of Cardiac Transcription Factor Nkx2.5 on Cardiomyoplasty Model in vitro
- NgR1 Expressed in P19 Embryonal Carcinoma Cells Differentiated by Retinoic Acid Can Activate STAT3
- 5-azacytidine induces cardiac differentiation of P19 embryonic stem cells
- Induction of Embryonal Teratocarcinoma Cell(P19 cell) to Differentiate into Smooth Muscle Cell