Korean J Physiol Pharmacol.
2014 Apr;18(2):129-134. 10.4196/kjpp.2014.18.2.129.
Alteration of Striatal Tetrahydrobiopterin in Iron-Induced Unilateral Model of Parkinson's Disease
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Dankook University, Cheonan 330-714, Korea. hgkimm@dankook.ac.kr
- 2Translational Research Center, Institute of Bio-Science Technology, Dankook University, Cheonan 330-714, Korea.
- KMID: 2285501
- DOI: http://doi.org/10.4196/kjpp.2014.18.2.129
Abstract
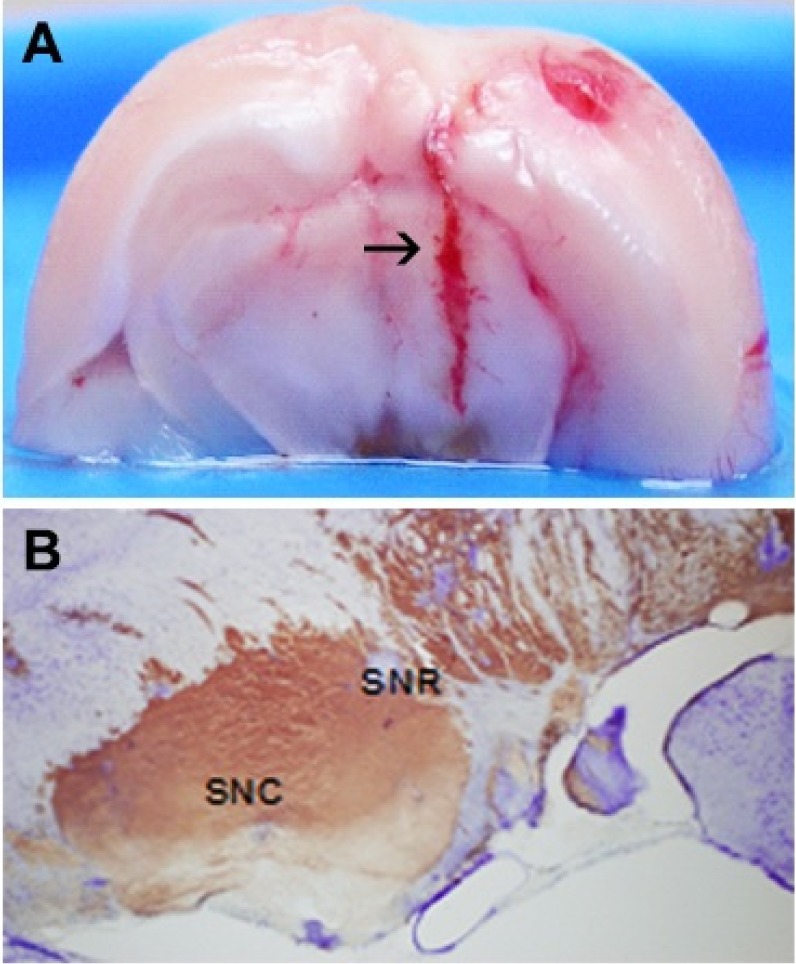
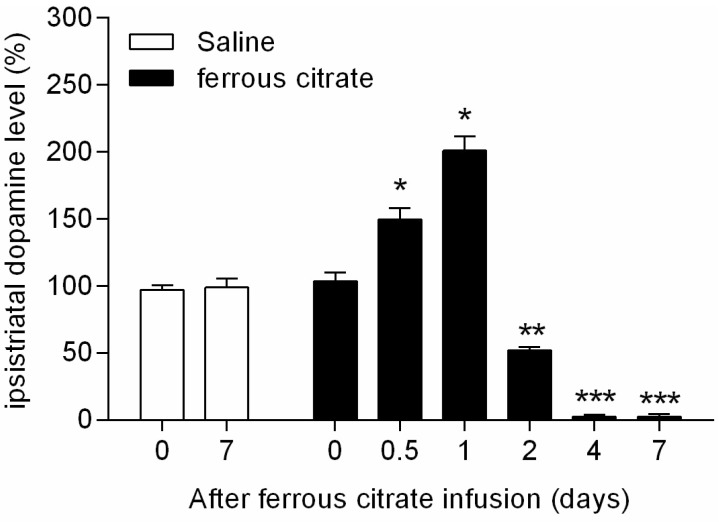
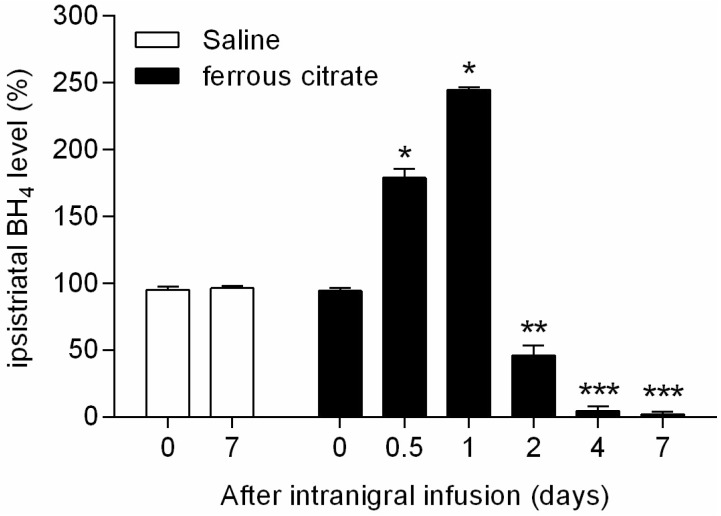
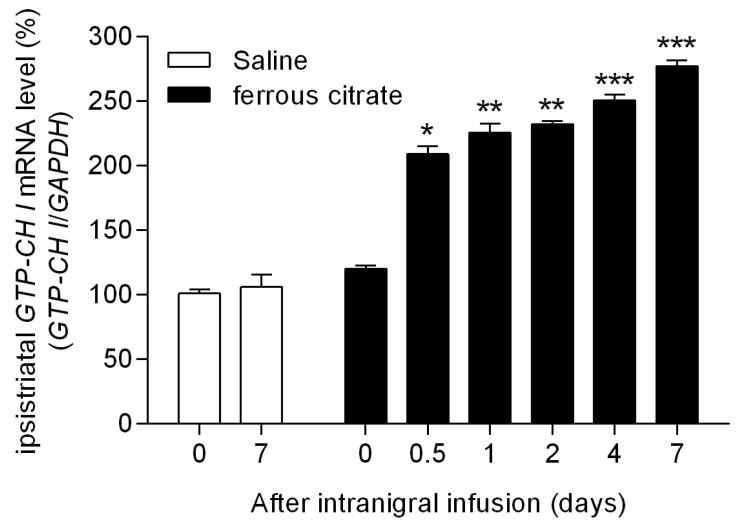
- It has been suggested that transition metal ions such as iron can produce an oxidative injuries to nigrostriatal dopaminergic neurons, like Parkinson's disease (PD) and subsequent compensative increase of tetrahydrobiopterin (BH4) during the disease progression induces the aggravation of dopaminergic neurodegeneration in striatum. It had been established that the direct administration of BH4 into neuron would induce the neuronal toxicity in vitro. To elucidate a role of BH4 in pathogenesis in the PD in vivo, we assessed the changes of dopamine (DA) and BH4 at striatum in unilateral intranigral iron infused PD rat model. The ipsistriatal DA and BH4 levels were significantly increased at 0.5 to 1 d and were continually depleting during 2 to 7 d after intranigral iron infusion. The turnover rate of BH4 was higher than that of DA in early phase. However, the expression level of GTP-cyclohydrolase I mRNA in striatum was steadily increased after iron administration. These results suggest that the accumulation of intranigral iron leads to generation of oxidative stress which damage to dopaminergic neurons and causes increased release of BH4 in the dopaminergic neuron. The degenerating dopaminergic neurons decrease the synthesis and release of both BH4 and DA in vivo that are relevance to the progression of PD. Based on these data, we propose that the increase of BH4 can deteriorate the disease progression in early phase of PD, and the inhibition of BH4 increase could be a strategy for PD treatment.
MeSH Terms
Figure
Reference
-
1. Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol Dis. 2000; 7:240–250. PMID: 10964596.
Article2. Hwang O, Choi HJ, Park SY. Up-regulation of GTP cyclohydrolase I and tetrahydrobiopterin by calcium influx. Neuroreport. 1999; 10:3611–3614. PMID: 10619653.
Article3. Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson\'s disease. Ann N Y Acad Sci. 2003; 991:120–131. PMID: 12846981.
Article4. Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson's disease. IUBMB Life. 2001; 52:135–141. PMID: 11798025.
Article5. Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol. 1997; 12:25–31. PMID: 9046040.6. Kingsbury AE, Mardsen CD, Foster OJ. DNA fragmentation in human substantia nigra: apoptosis or perimortem effect? Mov Disord. 1998; 13:877–884. PMID: 9827610.
Article7. Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003; 53(Suppl 3):S61–S70. discussion S70-72. PMID: 12666099.
Article8. McNaught KS, Olanow CW. Proteolytic stress: a unifying concept for the etiopathogenesis of Parkinson's disease. Ann Neurol. 2003; 53(Suppl 3):S73–S84. discussion S84-86. PMID: 12666100.
Article9. Hwang O, Baker H, Gross S, Joh TH. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998; 28:140–153. PMID: 9450514.
Article10. Kaufman S. New tetrahydrobiopterin-dependent systems. Annu Rev Nutr. 1993; 13:261–286. PMID: 8103664.
Article11. Nagatsu I, Ichinose H, Sakai M, Titani K, Suzuki M, Nagatsu T. Immunocytochemical localization of GTP cyclohydrolase I in the brain, adrenal gland, and liver of mice. J Neural Transm Gen Sect. 1995; 102:175–188. PMID: 8788067.
Article12. Offen D, Ziv I, Sternin H, Melamed E, Hochman A. Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson's disease. Exp Neurol. 1996; 141:32–39. PMID: 8797665.
Article13. Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson's disease. Neurotox Res. 2003; 5:165–176. PMID: 12835121.
Article14. Hastings TG, Zigmond MJ. Loss of dopaminergic neurons in parkinsonism: possible role of reactive dopamine metabolites. J Neural Transm Suppl. 1997; 49:103–110. PMID: 9266419.
Article15. Weingarten P, Zhou QY. Protection of intracellular dopamine cytotoxicity by dopamine disposition and metabolism factors. J Neurochem. 2001; 77:776–785. PMID: 11331406.
Article16. Choi HJ, Jang YJ, Kim HJ, Hwang O. Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol Pharmacol. 2000; 58:633–640. PMID: 10953058.
Article17. Choi HJ, Kim SW, Lee SY, Hwang O. Dopamine-dependent cytotoxicity of tetrahydrobiopterin: a possible mechanism for selective neurodegeneration in Parkinson's disease. J Neurochem. 2003; 86:143–152. PMID: 12807434.
Article18. Davis MD, Kaufman S. Products of the tyrosine-dependent oxidation of tetrahydrobiopterin by rat liver phenylalanine hydroxylase. Arch Biochem Biophys. 1993; 304:9–16. PMID: 8323303.
Article19. Davis MD, Kaufman S, Milstien S. The auto-oxidation of tetrahydrobiopterin. Eur J Biochem. 1988; 173:345–351. PMID: 3360013.
Article20. Kirsch M, Korth HG, Stenert V, Sustmann R, de Groot H. The autoxidation of tetrahydrobiopterin revisited. Proof of superoxide formation from reaction of tetrahydrobiopterin with molecular oxygen. J Biol Chem. 2003; 278:24481–24490. PMID: 12714605.21. Thoeni G, Werner ER, Werner-Felmayer G. Tetrahydropteridines suppress gene expression and induce apoptosis of activated RAW264.7 cells via formation of hydrogen peroxide. Free Radic Biol Med. 2004; 37:375–385. PMID: 15223071.
Article22. Choi HJ, Lee SY, Cho Y, Hwang O. Inhibition of vesicular monoamine transporter enhances vulnerability of dopaminergic cells: relevance to Parkinson's disease. Neurochem Int. 2005; 46:329–335. PMID: 15707697.
Article23. Double KL, Ben-Shachar D, Youdim MB, Zecca L, Riederer P, Gerlach M. Influence of neuromelanin on oxidative pathways within the human substantia nigra. Neurotoxicol Teratol. 2002; 24:621–628. PMID: 12200193.
Article24. Lentz SI, Kapatos G. Tetrahydrobiopterin biosynthesis in the rat brain: heterogeneity of GTP cyclohydrolase I mRNA expression in monoamine-containing neurons. Neurochem Int. 1996; 28:569–582. PMID: 8792338.
Article25. Keane PC, Kurzawa M, Blain PG, Morris CM. Mitochondrial dysfunction in Parkinson's disease. Parkinsons Dis. 2011; 2011:716871. PMID: 21461368.
Article26. Eşrefoğlu M. Cell injury and death: Oxidative stress and antioxidant defense system: Review. Turkiye Klinikleri J Med Sci. 2009; 29:1660–1676.27. Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Compact: The Coronal Plates and Diagrams. Allen Institute: Academic Press;2008.28. Zhao Y, Cao J, Chen YS, Zhu Y, Patrick C, Chien B, Cheng A, Foehr ED. Detection of tetrahydrobiopterin by LC-MS/MS in plasma from multiple species. Bioanalysis. 2009; 1:895–903. PMID: 21083061.
Article29. Mokrý J. Experimental models and behavioural tests used in the study of Parkinson's disease. Physiol Res. 1995; 44:143–150. PMID: 8869270.30. Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991; 114:1953–1975. PMID: 1832073.
Article31. Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994; 63:793–807. PMID: 7519659.
Article32. Morris CM, Keith AB, Edwardson JA, Pullen RG. Uptake and distribution of iron and transferrin in the adult rat brain. J Neurochem. 1992; 59:300–306. PMID: 1613505.
Article33. Ryan BJ, Lourenço-Venda LL, Crabtree MJ, Hale AB, Channon KM, Wade-Martins R. α-Synuclein and mitochondrial bioenergetics regulate tetrahydrobiopterin levels in a human dopaminergic model of Parkinson disease. Free Radic Biol Med. 2014; 67:58–68. PMID: 24148766.
Article34. Lee KS, Lee JK, Kim HG, Kim HR. Differential Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on Motor Behavior and Dopamine Levels at Brain Regions in Three Different Mouse Strains. Korean J Physiol Pharmacol. 2013; 17:89–97. PMID: 23440908.
Article35. Lotharius J, O'Malley KL. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem. 2000; 275:38581–38588. PMID: 10969076.36. Choe MA, Koo BS, An GJ, Jeon S. Effects of Treadmill Exercise on the Recovery of Dopaminergic Neuron Loss and Muscle Atrophy in the 6-OHDA Lesioned Parkinson's Disease Rat Model. Korean J Physiol Pharmacol. 2012; 16:305–312. PMID: 23129977.
Article37. Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992; 32:804–812. PMID: 1471873.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Striatal Network Model in Parkinson Disease: Preliminary Study
- Alteration of nigral iron and ferritin in 6-hydroxydopamine rat parkinsonian model
- Neural Network Model of Basal Ganglia: Preliminary Study
- Changes of Dopaminergic Neurons in Parkinson's Rat Model after Fetal Striatal Transplantation
- Subthalamic Lesion Protects Nigral Dopaminergic Neurons from 6-Hydroxydopamine-induced Cytotoxicity in the Rat Model of Early Parkinson's Disease