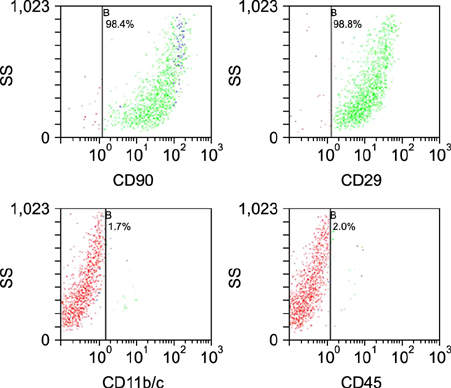
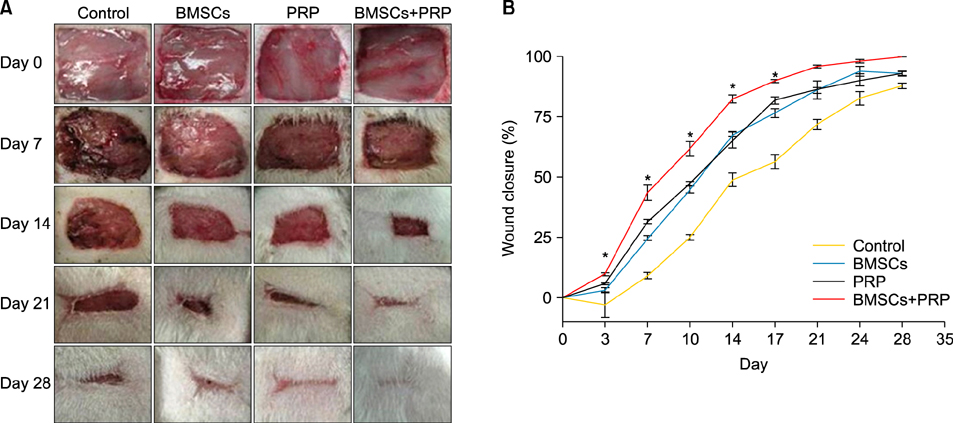
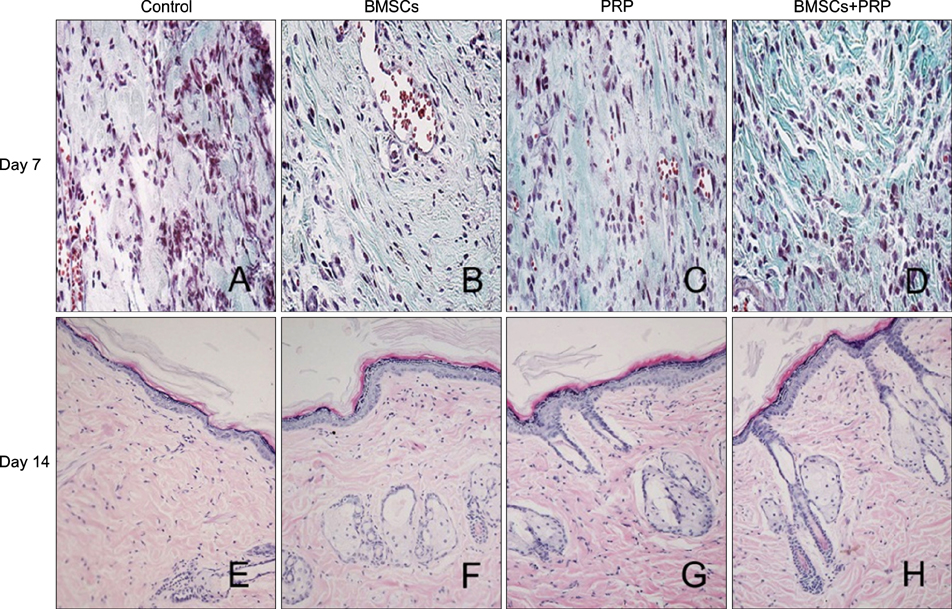
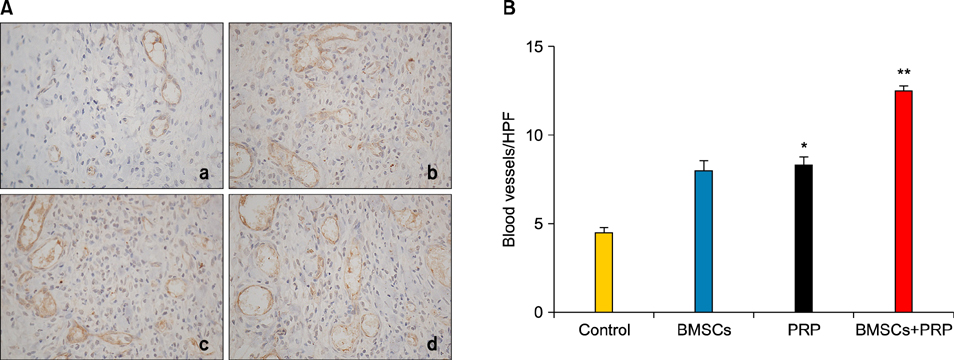
Ann Dermatol.
2014 Feb;26(1):1-10. 10.5021/ad.2014.26.1.1.
Synergistic Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1School of Pharmacy, Wenzhou Medical College, Campus of Chashan High Education, Wenzhou, China. shaotsiang@163.com
- 2Department of Endocrinology, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- KMID: 2265691
- DOI: http://doi.org/10.5021/ad.2014.26.1.1
Abstract
- BACKGROUND
Diabetic wounds are a major clinical challenge, because minor skin wounds can lead to chronic, unhealed ulcers and ultimately result in infection, gangrene, or even amputation. Studies on bone marrow derived mesenchymal stem cells (BMSCs) and a series of growth factors have revealed their many benefits for wound healing and regeneration. Platelet-rich plasma (PRP) may improve the environment for BMSC development and differentiation. However, whether combined use of BMSCs and PRP may be more effective for accelerating diabetic ulcer healing remains unclear.
OBJECTIVE
We investigated the efficacy of BMSCs and PRP for the repair of refractory wound healing in a diabetic rat model.
METHODS
Forty-eight rats with diabetes mellitus induced by streptozotocin were divided into four groups: treatment with BMSCs plus PRP, BMSCs alone, PRP alone, phosphate buffered saline. The rate of wound closure was quantified. A histopathological study was conducted regarding wound depth and the skin edge at 7, 14, and 28 days after surgery.
RESULTS
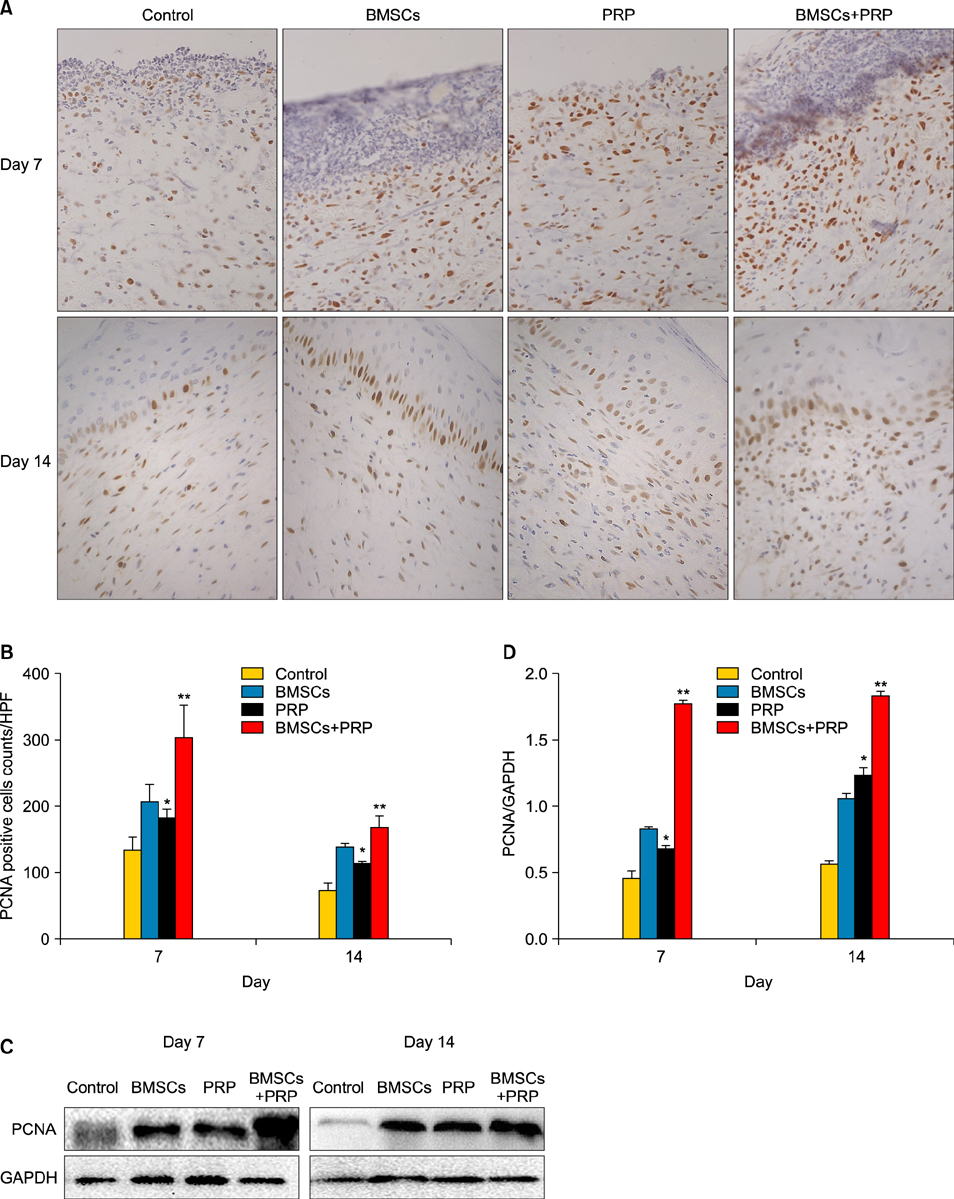
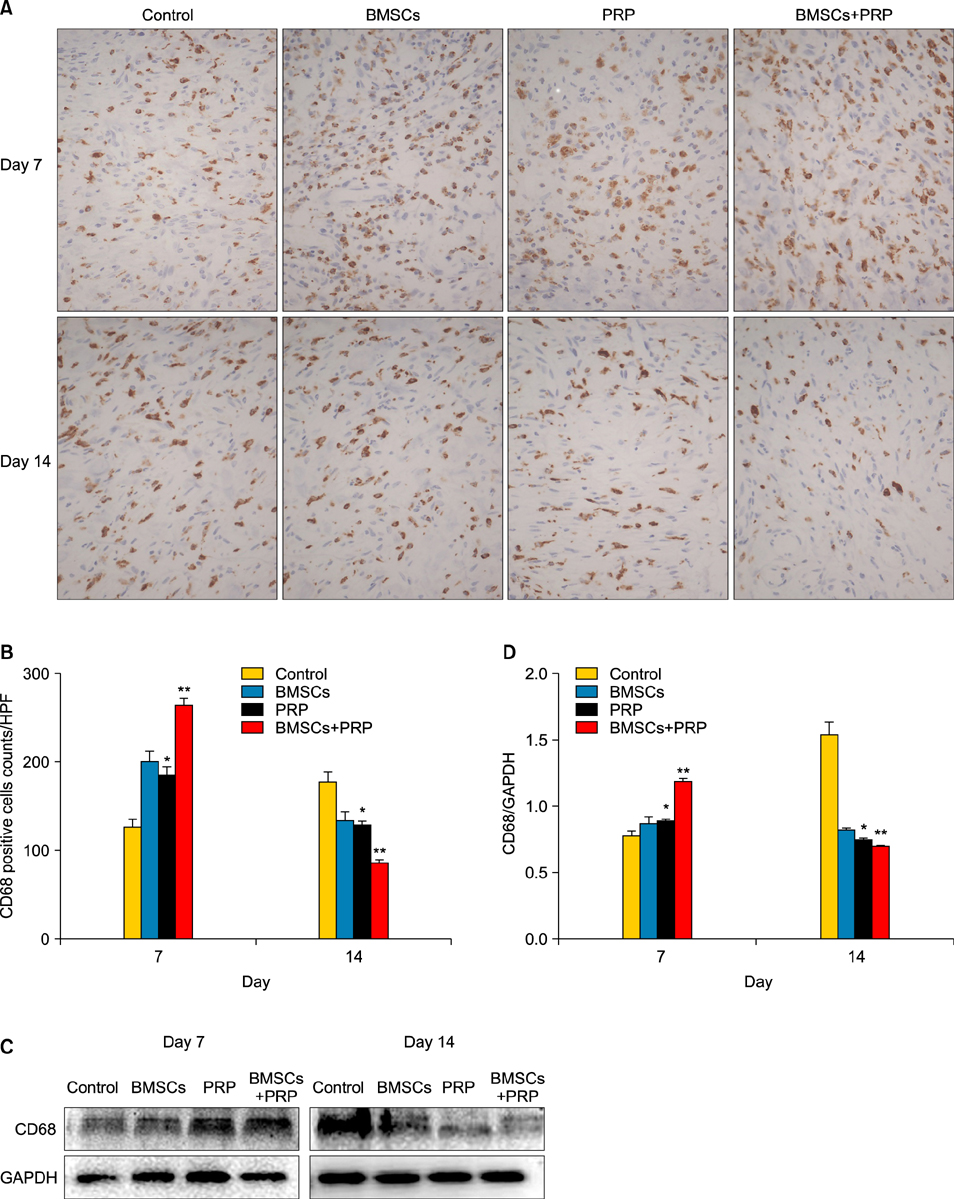
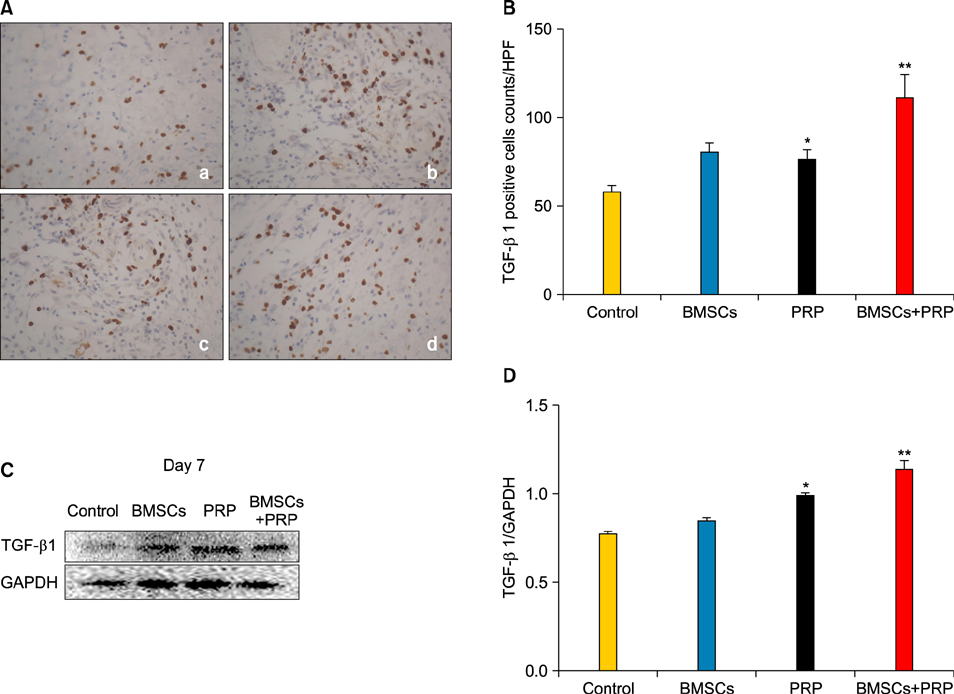
Wound healing rates were significantly higher in the BMSC plus PRP group than in the other groups. The immunohistochemistry results showed that the expression of platelet/endothelial cell adhesion molecule 1, proliferating cell nuclear antigen, and transforming growth factor-beta1 increased significantly in the BMSC plus PRP group compared to the other treatment groups. On day 7, CD68 expression increased significantly in the wounds of the BMSC plus PRP group, but decreased markedly at day 14 compared to the controls.
CONCLUSION
The combination of BMSCs and PRP aids diabetic wound repair and regeneration.
MeSH Terms
-
Amputation
Animals
Bone Marrow
Cell Adhesion
Diabetes Mellitus
Gangrene
Immunohistochemistry
Intercellular Signaling Peptides and Proteins
Mesenchymal Stromal Cells*
Models, Animal
Platelet-Rich Plasma*
Proliferating Cell Nuclear Antigen
Rats*
Regeneration
Skin
Streptozocin
Ulcer
Wound Healing
Wounds and Injuries
Intercellular Signaling Peptides and Proteins
Proliferating Cell Nuclear Antigen
Streptozocin
Figure
Reference
-
1. Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2012; 96:1–9.
Article2. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007; 117:1219–1222.
Article3. Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997; 276:75–81.
Article4. Ansurudeen I, Sunkari VG, Grünler J, Peters V, Schmitt CP, Catrina SB, et al. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids. 2012; 43:127–134.
Article5. Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J. 2008; 5:453–463.
Article6. Schneider RK, Anraths J, Kramann R, Bornemann J, Bovi M, Knüchel R, et al. The role of biomaterials in the direction of mesenchymal stem cell properties and extracellular matrix remodelling in dermal tissue engineering. Biomaterials. 2010; 31:7948–7959.
Article7. Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008; 103:525–536.
Article8. Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011; 8:307–312.
Article9. Frykberg RG, Driver VR, Carman D, Lucero B, Borris-Hale C, Fylling CP, et al. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy Wound Manage. 2010; 56:36–44.10. Kuo YR, Goto S, Shih HS, Wang FS, Lin CC, Wang CT, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009; 87:1769–1777.
Article11. Akela A, Nandi SK, Banerjee D, Das P, Roy S, Joardar SN, et al. Evaluation of autologous bone marrow in wound healing in animal model: a possible application of autologous stem cells. Int Wound J. 2012; 9:505–516.
Article12. Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care. 2005; 18:491–500.
Article13. Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, et al. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol. 2008; 180:1598–1608.
Article14. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 114:1502–1508.
Article15. Demidova-Rice TN, Wolf L, Deckenback J, Hamblin MR, Herman IM. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS One. 2012; 7:e32146.
Article16. Raza H, Prabu SK, John A, Avadhani NG. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int J Mol Sci. 2011; 12:3133–3147.
Article17. Wang A, Ding X, Sheng S, Yao Z. Bone morphogenetic protein receptor in the osteogenic differentiation of rat bone marrow stromal cells. Yonsei Med J. 2010; 51:740–745.
Article18. Carter CA, Jolly DG, Worden CE Sr, Hendren DG, Kane CJ. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003; 74:244–255.
Article19. Bir SC, Esaki J, Marui A, Sakaguchi H, Kevil CG, Ikeda T, et al. Therapeutic treatment with sustained-release platelet-rich plasma restores blood perfusion by augmenting ischemia-induced angiogenesis and arteriogenesis in diabetic mice. J Vasc Res. 2011; 48:195–205.
Article20. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011; 31:212–218.
Article21. Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005; 26:306–319.
Article22. Lee YH, Chang JJ, Chien CT, Yang MC, Chien HF. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Exp Diabetes Res. 2012; 2012:504693.
Article23. Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010; 31:467–480.
Article24. Ganeshkumar M, Ponrasu T, Krithika R, Iyappan K, Gayathri VS, Suguna L. Topical application of Acalypha indica accelerates rat cutaneous wound healing by up-regulating the expression of Type I and III collagen. J Ethnopharmacol. 2012; 142:14–22.
Article25. Elsharawy MA, Naim M, Greish S. Human CD34+ stem cells promote healing of diabetic foot ulcers in rats. Interact Cardiovasc Thorac Surg. 2012; 14:288–293.
Article26. Roedersheimer M, Nijmeh H, Burns N, Sidiakova AA, Stenmark KR, Gerasimovskaya EV. Complementary effects of extracellular nucleotides and platelet-derived extracts on angiogenesis of vasa vasorum endothelial cells in vitro and subcutaneous Matrigel plugs in vivo. Vasc Cell. 2011; 3:4.
Article27. Ting AE, Mays RW, Frey MR, Hof WV, Medicetty S, Deans R. Therapeutic pathways of adult stem cell repair. Crit Rev Oncol Hematol. 2008; 65:81–93.
Article28. Atiba A, Nishimura M, Kakinuma S, Hiraoka T, Goryo M, Shimada Y, et al. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am J Surg. 2011; 201:809–818.
Article29. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012; 49:35–43.
Article30. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006; 34:1774–1778.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Using Bone Marrow Mesenchymal Stem Cells Versus Platelet Rich Plasma on the Healing of Induced Oral Ulcer in Albino Rats
- Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
- The Effect of In Vivo Mobilization of Bone Marrow Stem Cells on the Pancreas of Diabetic Albino Rats (A Histological & Immunohistochemical Study)
- Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix
- Protective effect of platelet-rich plasma against cold ischemia-induced apoptosis of canine adipose-derived mesenchymal stem cells