J Vet Sci.
2015 Sep;16(3):381-384. 10.4142/jvs.2015.16.3.381.
Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix
- Affiliations
-
- 1Laboratory of Animal Virology and Cell Culture, College of Veterinary Medicine, UNESP-Universidade Estadual Paulista, Aracatuba, SP 16050-680, Brazil. tcardoso@fmva.unesp.br
- 2Small Animal Surgery Center, College of Veterinary Medicine, UNESP-Universidade Estadual Paulista, Aracatuba, SP 16050-680, Brazil.
- KMID: 2344312
- DOI: http://doi.org/10.4142/jvs.2015.16.3.381
Abstract
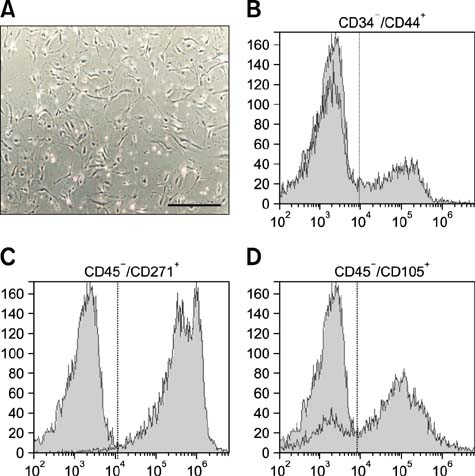
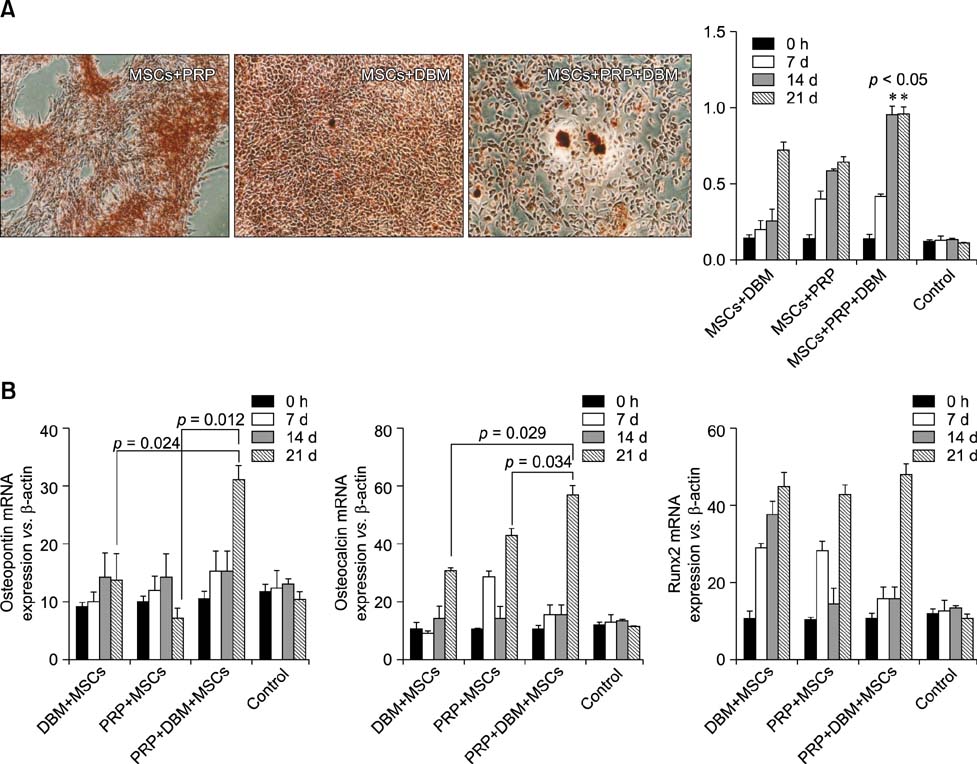
- Canine mesenchymal cells (MSCs) derived from Wharton's jelly were co-cultured, then supplemented or not supplemented with platelet rich plasma (PRP) and demineralized bone matrix (DBM) to verify osteogenic differentiation. Osteoblastic differentiation followed by mineralized bone matrix production was found to be significantly higher (p < 0.05) when MSCs were associated with PRP/DBM in culture after 14-21-days of induction. Osteopontin and osteocalcin gene expression were significantly superior (p < 0.05) under the same culture conditions after 21 days of observation. In conclusion, addition of PRP to DBM co-cultured with MSCs successfully induced osteogenesis in vitro.
MeSH Terms
Figure
Reference
-
1. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004; 91:4–15.
Article2. Cardoso TC, Ferrari HF, Garcia AF, Novais JB, Silva-Frade C, Ferrarezi MC, Andrade AL, Gameiro R. Isolation and characterization of Wharton's jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012; 12:18.
Article3. Chaudhary LR, Hofmeister AM, Hruska KA. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone. 2004; 34:402–411.
Article4. Chung DJ, Hayashi K, Toupadakis CA, Wong A, Yellowley CE. Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res Vet Sci. 2012; 92:66–75.
Article5. Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008; 92:161–168.
Article6. Filioli Uranio MF, Valentini L, Lange-Consiglio A, Caira M, Guaricci AC, L'Abbate A, Catacchio CR, Ventura M, Cremonesi F, Dell'Aquila ME. Isolation, proliferation, cytogenetic, and molecular characterization and in vitro differentiation potency of canine stem cells from foetal adnexa: a comparative study by amniotic fluid, amnion; and umbilical cord matrix. Mol Reprod Dev. 2011; 78:361–373.
Article7. Handschel J, Naujoks C, Langenbach F, Berr K, Depprich RA, Ommerborn MA, Kübler NR, Brinkmann M, Kögler G, Meyer U. Comparison of ectopic bone formation of embryonic stem cells and cord blood stem cells in vivo. Tissue Eng Part A. 2010; 16:2475–2483.
Article8. Henderson JL, Cupp CL, Ross EV, Shick PC, Keefe MA, Wester DC, Hannon T, McConnell D. The effects of autologous platelet gel on wound healing. Ear Nose Throat J. 2003; 82:598–602.
Article9. Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol (Paris). 2009; 57:318–323.
Article10. Ishida K, Acharya C, Christiansen BA, Yik JHN, DiCesare PE, Haudenschild DR. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone. 2013; 55:23–35.
Article11. Kärner E, Unger C, Sloan AJ, Ährlund-Richter L, Sugars RV, Wendel M. Bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Stem Cells Dev. 2007; 16:39–52.
Article12. Park SB, Seo MS, Kim HS, Kang KS. Isolation and characterization of canine amniotic membrane-derived multipotent stem cells. PLoS One. 2012; 7:e44693.
Article13. Pradeep AR, Shetty SK, Garg G, Pai S. Clinical effectiveness of autologous platelet-rich plasma and peptide-enhanced bone graft in the treatment of intrabony defects. J Periodontol. 2009; 80:62–71.
Article14. Seo MS, Jeong YH, Park JR, Park SB, Rho KH, Kim HS, Yu KR, Lee SH, Jung JW, Lee YS, Kang KS. Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells. J Vet Sci. 2009; 10:181–187.
Article15. Souza TF, Andrade AL, Ferreira GT, Sakamoto SS, Albuquerque VB, Bonfim SRM, Luvizotto MC, Louzada MJ. Healing and expression of growth factors (TGF-β and PDGF) in canine radial ostectomy gap containing plateletrich plasma. Vet Comp Orthop Traumatol. 2012; 25:445–452.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- The quantitative analysis by digital subtraction radiography on the effect of Enamel Matrix Protein and Platelet-Rich Plasma, combined with Xenograft in the treatment of intrabony defect in humans
- Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation