Int J Stem Cells.
2019 Mar;12(1):95-106. 10.15283/ijsc18074.
The Effect of Using Bone Marrow Mesenchymal Stem Cells Versus Platelet Rich Plasma on the Healing of Induced Oral Ulcer in Albino Rats
- Affiliations
-
- 1Oral Biology Department, Faculty of Dentistry, Damanhour University, Damanhour, Egypt. fatma.rashed@dnt.dmu.edu.eg
- 2Oral Biology Department, Faculty of Dentistry, Tanta University, Tanta, Egypt.
- KMID: 2447228
- DOI: http://doi.org/10.15283/ijsc18074
Abstract
- BACKGROUND AND OBJECTIVES
Oral ulceration is one of the most common debilitating condition that affects the oral cavity. In this study, the effect of locally injected platelet rich plasma (PRP) and bone marrow-derived mesenchymal stem cells (BMSCs) on the healing of oral ulcer was investigated.
METHODS AND RESULTS
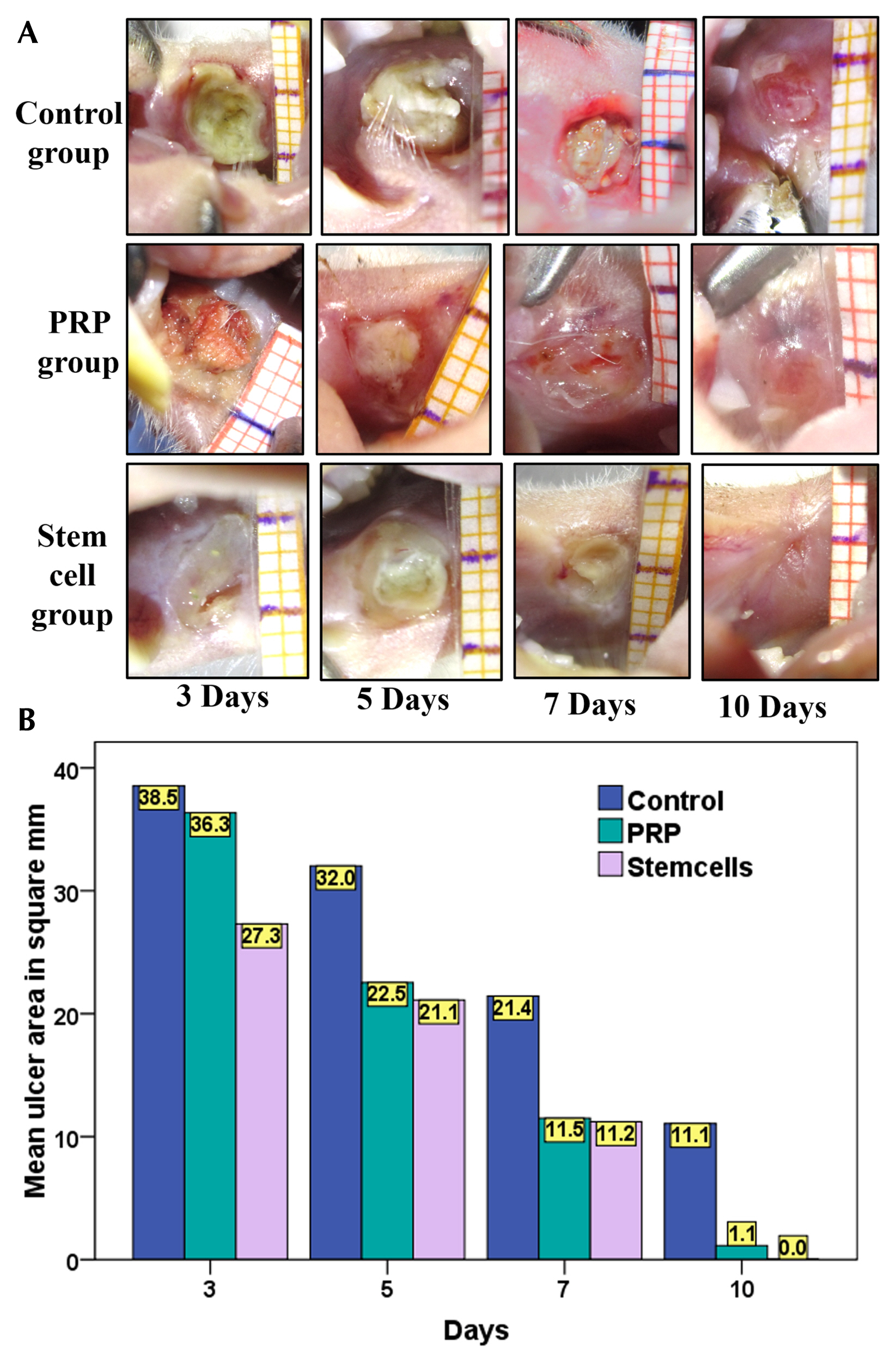
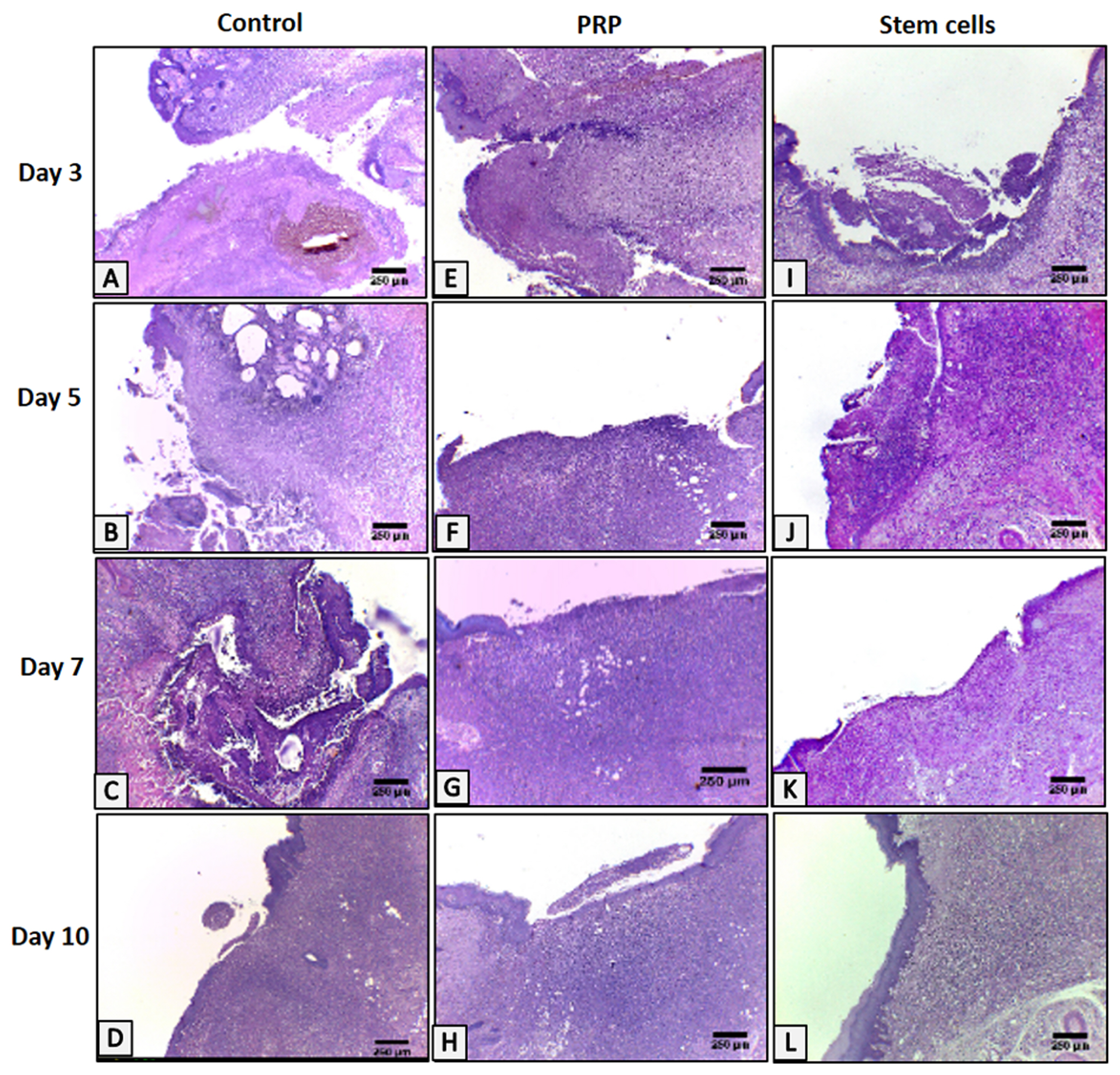
An ulcer was induced in buccal mucosa of rats by using 5mm biopsy punch followed by application of cotton swab soaked with formocresol for 60sec. The ulcer was left untreated in the control group, treated with intralesional injection of PRP, or isolated cultured BMSCs. Data were analyzed clinically, histologically and immunohistologically on day 3, 5, 7 and 10. BMSCs group showed smaller ulcer area throughout the whole experimental period than the other groups with complete resolution of the ulcer on day 10, unlike the control group. However, there was no significant difference with PRP, on day 5, 7 and 10, regarding clinical ulcer size. BMSCs group showed better histological results regarding the rate of epithelial cell migration, the number of inflammatory cells, thickness and organization of collagen fibres and the number of blood vessels, with complete re-epithelization on day 10. BMSCs group showed a greater number of anti-PCNA positive nuclei throughout the whole experimental period than the other groups except on day 5, PRP had higher mean numbers of anti-PCNA positive nuclei in both tissues.
CONCLUSIONS
Both PRP and BMSCs accelerate wound healing and enhance the quality of the healing tissue with the latter being slightly more effective and faster.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Schneider LC, Schneider AE. Diagnosis of oral ulcers. Mt Sinai J Med. 1998; 65:383–387. PMID: 9844367.2. Scully C, Shotts R. ABC of oral health. Mouth ulcers and other causes of orofacial soreness and pain. BMJ. 2000; 321:162–165. DOI: 10.1136/bmj.321.7254.162. PMID: 10894697. PMCID: 1118165.
Article3. Alamoudi NM, El Ashiry EA, Farsi NM, El Derwi DA, Atta HM. Treatment of oral ulcers in dogs using adipose tissue-derived mesenchymal stem cells. J Clin Pediatr Dent. 2014; 38:215–222. DOI: 10.17796/jcpd.38.3.193115427jg6vl60. PMID: 25095315.
Article4. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003; 83:835–870. DOI: 10.1152/physrev.2003.83.3.835. PMID: 12843410.
Article5. Arora NS, Ramanayake T, Ren YF, Romanos GE. Plateletrich plasma: a literature review. Implant Dent. 2009; 18:303–310. DOI: 10.1097/ID.0b013e31819e8ec6. PMID: 19667818.
Article6. Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010; 316:2213–2219. DOI: 10.1016/j.yexcr.2010.05.009. PMID: 20471978. PMCID: 2902653.
Article7. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008; 14:249–258. DOI: 10.1089/ten.teb.2008.0062. PMID: 18601587.
Article8. Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004; 30:145–151. DOI: 10.1016/j.transci.2004.01.004. PMID: 15062754.
Article9. Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004; 10:955–964. DOI: 10.1089/1076327041348284. PMID: 15265313.
Article10. Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010; 125:510–516. DOI: 10.1097/PRS.0b013e3181c722bb. PMID: 20124836. PMCID: 4076140.
Article11. Marfia G, Navone SE, Di Vito C, Ughi N, Tabano S, Miozzo M, Tremolada C, Bolla G, Crotti C, Ingegnoli F, Rampini P, Riboni L, Gualtierotti R, Campanella R. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015; 11:183–206. DOI: 10.1080/15476278.2015.1126018. PMID: 26652928. PMCID: 4879897.
Article12. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed. Washington: National Academy Press;2011. p. 41–196.13. Duarte CM, Quirino MR, Patrocínio MC, Anbinder AL. Effects of Chamomilla recutita (L.) on oral wound healing in rats. Med Oral Patol Oral Cir Bucal. 2011; 16:e716–e721. PMID: 21196867.14. de Carvalho FB, Andrade AS, Rasquin LC, de Castro IV, Cangussu MC, Pinheiro AL, dos Santos JN. Effect of laser (λ 660 nm) and LED (λ 630 nm) photobiomodulation on formocresol-induced oral ulcers: a clinical and histological study on rodents. Lasers Med Sci. 2015; 30:389–396. DOI: 10.1007/s10103-014-1680-7.
Article15. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007; 13:1299–1312. DOI: 10.1089/ten.2006.0278. PMID: 17518741.
Article16. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014; 6:265sr6. DOI: 10.1126/scitranslmed.3009337. PMID: 25473038. PMCID: 4973620.
Article17. El-Menoufy H, Aly LA, Aziz MT, Atta HM, Roshdy NK, Rashed LA, Sabry D. The role of bone marrow-derived mesenchymal stem cells in treating formocresol induced oral ulcers in dogs. J Oral Pathol Med. 2010; 39:281–289. DOI: 10.1111/j.1600-0714.2009.00819.x. PMID: 19804505.
Article18. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004; 91:4–15. DOI: 10.1160/TH03-07-0440. PMID: 14691563.
Article19. Aiba-Kojima E, Tsuno NH, Inoue K, Matsumoto D, Shigeura T, Sato T, Suga H, Kato H, Nagase T, Gonda K, Koshima I, Takahashi K, Yoshimura K. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture. Wound Repair Regen. 2007; 15:511–520. DOI: 10.1111/j.1524-475X.2007.00259.x. PMID: 17650095.
Article20. Everts PA, Brown Mahoney C, Hoffmann JJ, Schönberger JP, Box HA, van Zundert A, Knape JT. Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors. 2006; 24:165–171. DOI: 10.1080/08977190600821327. PMID: 17079200.
Article21. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005; 84:434–439. DOI: 10.1177/154405910508400507. PMID: 15840779.
Article22. Sinno H, Prakash S. Complements and the wound healing cascade: an updated review. Plast Surg Int. 2013; 2013:146764. PMID: 23984063. PMCID: 3741993.
Article23. Mirastschijski U, Jokuszies A, Vogt PM. Skin wound healing: repair biology, wound and scar treatment. Warren R, Neligan P, editors. Plastic surgery. 3rd ed. Philadelphia, PA: Elsevier Saunders;2013. p. 271–273.24. Carter CA, Jolly DG, Worden CE Sr, Hendren DG, Kane CJ. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003; 74:244–255. DOI: 10.1016/S0014-4800(03)00017-0. PMID: 12782011.
Article25. Lindeboom JA, Mathura KR, Aartman IH, Kroon FH, Milstein DM, Ince C. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin Oral Implants Res. 2007; 18:133–139. DOI: 10.1111/j.1600-0501.2006.01288.x. PMID: 17224034.
Article26. Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Pannonica Adriat. 2007; 16:156–165. PMID: 18204746.27. Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010; 83:1–9. PMID: 20351977. PMCID: 2844688.28. DeRossi R, Coelho AC, Mello GS, Frazílio FO, Leal CR, Facco GG, Brum KB. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras. 2009; 24:276–281. DOI: 10.1590/S0102-86502009000400006. PMID: 19705027.
Article29. Wasterlain AS, Braun HJ, Dragoo JL. Contents and formulations of platelet rich plasma. Maffulli N, editor. Platelet rich plasma in musculoskeletal practice. London: Springer-Verlag;2016. p. 1–29.
Article30. Arnoczky SP, Sheibani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc Rev. 2013; 21:180–185. DOI: 10.1097/JSA.0b013e3182999712. PMID: 24212364.31. Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, Le AD. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012; 21:937–947. DOI: 10.1089/scd.2011.0252. PMID: 21689066. PMCID: 3315752.
Article32. I T, Sumita Y, Minamizato T, Umebayashi M, Liu Y, Tran SD, Asahina I. Bone marrow-derived cell therapy for oral mucosal repair after irradiation. J Dent Res. 2014; 93:813–820. DOI: 10.1177/0022034514541124. PMID: 24980658. PMCID: 4293762.
Article33. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008; 3:e1886. DOI: 10.1371/journal.pone.0001886. PMID: 18382669. PMCID: 2270908.
Article34. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008; 180:2581–2587. DOI: 10.4049/jimmunol.180.4.2581. PMID: 18250469.
Article35. Lin CY, Lee BS, Liao CC, Cheng WJ, Chang FM, Chen MH. Transdifferentiation of bone marrow stem cells into acinar cells using a double chamber system. J Formos Med Assoc. 2007; 106:1–7. DOI: 10.1016/S0929-6646(09)60209-6. PMID: 17282964.
Article36. Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006; 14:325–335. DOI: 10.1111/j.1743-6109.2006.00128.x. PMID: 16808812.
Article37. Jin IG, Kim JH, Wu HG, Hwang SJ. Effect of mesenchymal stem cells and platelet-derived growth factor on the healing of radiation induced ulcer in rats. Tissue Eng Regen Med. 2016; 13:78–90. DOI: 10.1007/s13770-015-0055-x. PMID: 30603388. PMCID: 6170994.
Article38. An Y, wei W, Jing H, Ming L, Liu S, Jin Y. Bone marrow mesenchymal stem cell aggregate: an optimal cell therapy for full-layer cutaneous wound vascularization and regeneration. Sci Rep. 2015; 5:17036. DOI: 10.1038/srep17036. PMID: 26594024. PMCID: 4655471.
Article39. Akino K, Mineda T, Akita S. Early cellular changes of human mesenchymal stem cells and their interaction with other cells. Wound Repair Regen. 2005; 13:434–440. DOI: 10.1111/j.1067-1927.2005.130411.x. PMID: 16008733.
Article40. Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004; 22:812–822. DOI: 10.1634/stemcells.22-5-812. PMID: 15342945. PMCID: 1388268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma in Streptozotocin-Induced Diabetic Rats
- Adipose Stem Cells as Alternatives for Bone Marrow Mesenchymal Stem Cells in Oral Ulcer Healing
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat
- The Possible Role of Mesenchymal Stem Cells Therapy in the Repair of Experimentally Induced Colitis in Male Albino Rats