Ann Dermatol.
2010 Aug;22(3):269-278. 10.5021/ad.2010.22.3.269.
The Roles of Reactive Oxygen Species Produced by Contact Allergens and Irritants in Monocyte-derived Dendritic Cells
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. mglee@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Dermatology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2265359
- DOI: http://doi.org/10.5021/ad.2010.22.3.269
Abstract
- BACKGROUND
Although reactive oxygen species (ROS) have been produced in both mouse bone marrow-derived dendritic cells (DCs) and XS-106 DCs by contact sensitizers and irritants in previous studies, the generation of ROS in human monocyte-derived DCs (MoDCs) and their role in contact hypersensitivity (CHS) has yet to be elucidated.
OBJECTIVE
The purpose of this study was to determine whether contact allergens and irritants induce ROS in MoDCs and, if so, to evaluate the role of contact allergen and irritant induced-ROS in MoDCs in CHS.
METHODS
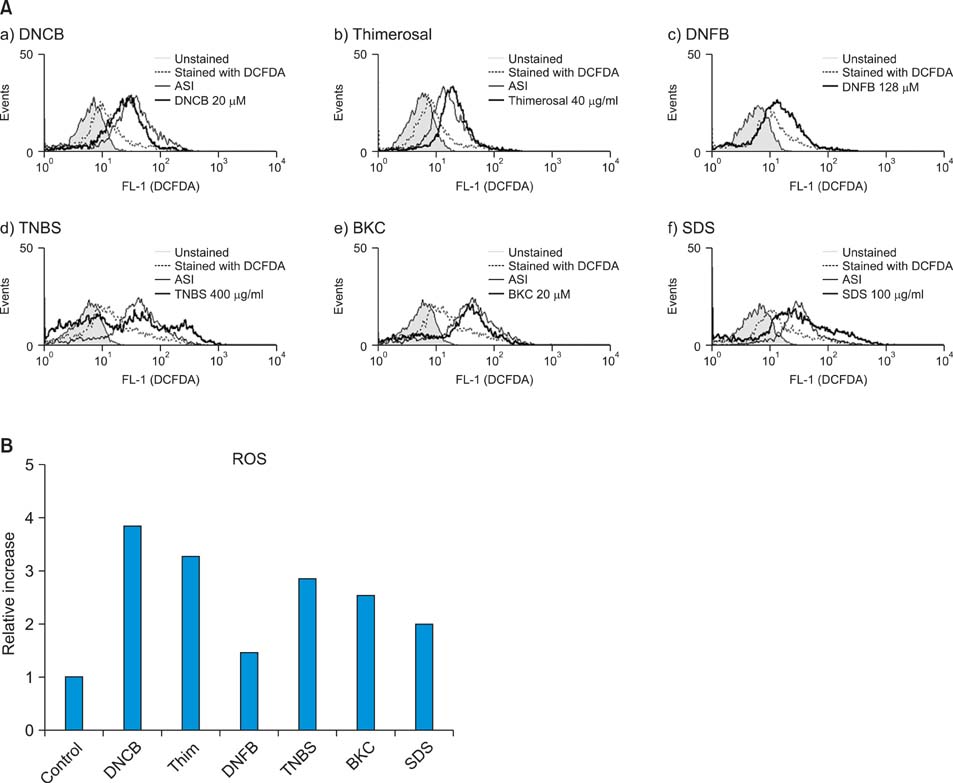
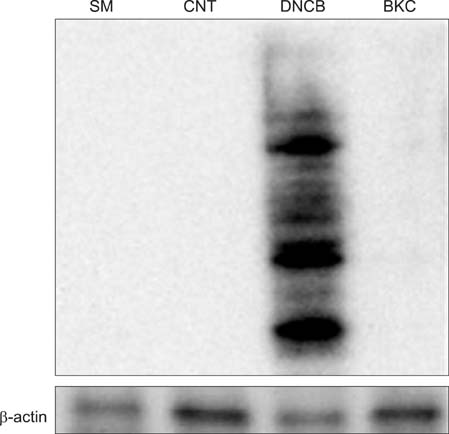
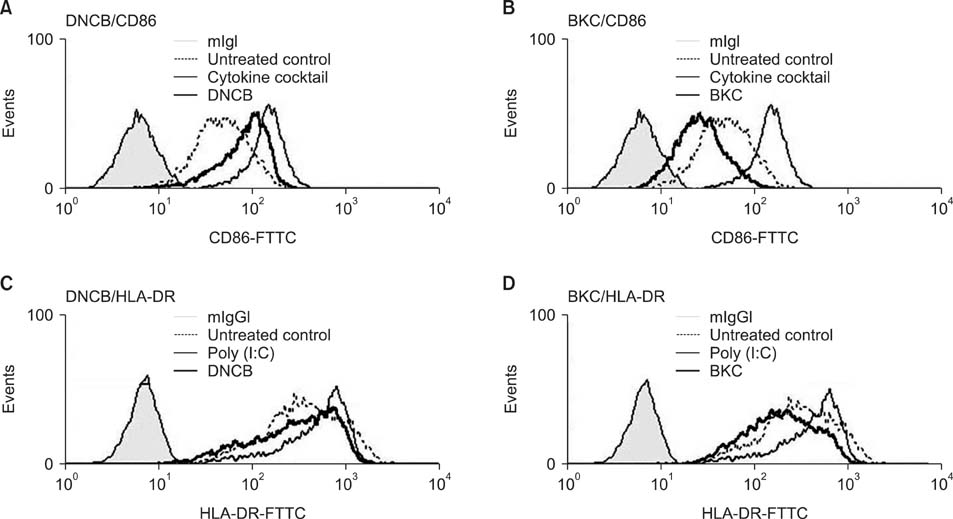
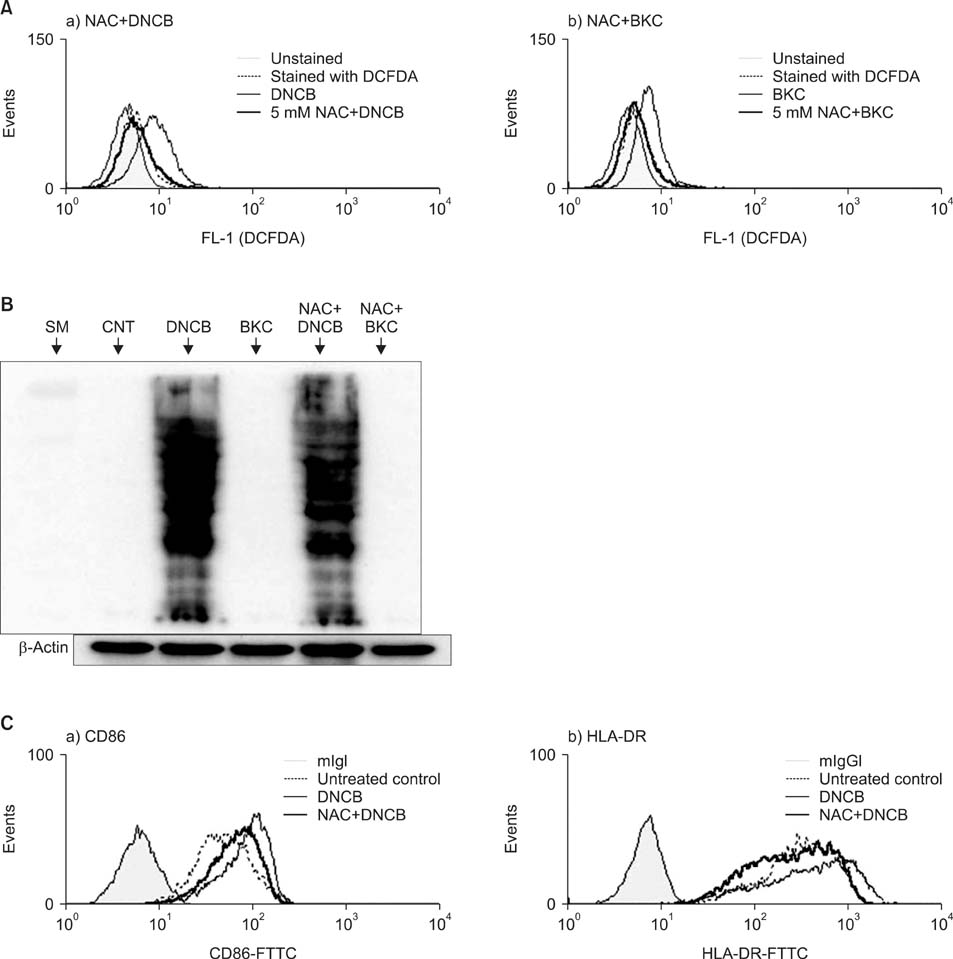
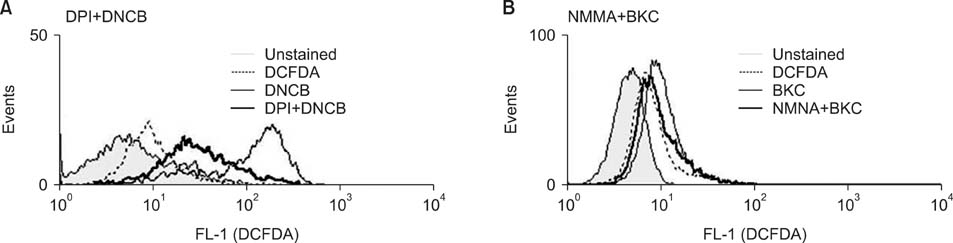
Production of ROS was measured by 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) assay. Surface CD86 and HLA-DR molecules were detected by flow cytometry. Protein carbonylation was detected by Western blotting.
RESULTS
ROS were produced by contact allergens such as dinitrochlorobenzene (DNCB) and thimerosal and the irritant benzalkonium chloride (BKC). DNCB-induced, but not BKC-induced, ROS increased surface CD86 and HLA-DR molecules on MoDCs and induced protein carbonylation. These changes were reduced in the presence of antioxidant N-acetyl cysteine.
CONCLUSION
Our results suggest that DNCB-induced ROS may be different from those induced by irritant BKC. The DNCB-induced ROS may be associated with the CHS response, because they activate surface molecules on DCs that are important for generating immune reactions.
Keyword
MeSH Terms
-
Allergens
Animals
Benzalkonium Compounds
Blotting, Western
Cysteine
Dendritic Cells
Dermatitis, Contact
Dinitrochlorobenzene
Flow Cytometry
HLA-DR Antigens
Humans
Irritants
Mice
Protein Carbonylation
Reactive Oxygen Species
Thimerosal
Allergens
Benzalkonium Compounds
Cysteine
Dinitrochlorobenzene
HLA-DR Antigens
Irritants
Reactive Oxygen Species
Thimerosal
Figure
Reference
-
1. Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008. 15:164–187.
Article2. Zabucchi G, Bellavite P, Berton G, Dri P. Free radicals generation by the inflammatory cells. Agents Actions Suppl. 1980. 7:159–166.
Article3. Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006. 40:1250–1258.
Article4. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000. 408:239–247.
Article5. Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006. 5:14.
Article6. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007. 39:44–84.
Article7. Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006. 126:2565–2575.
Article8. Bartosz G. Reactive oxygen species: destroyers or messengers? Biochem Pharmacol. 2009. 77:1303–1315.
Article9. Je JH, Lee TH, Kim DH, Cho YH, Lee JH, Kim SC, et al. Mitochondrial ATP synthase is a target for TNBS-induced protein carbonylation in XS-106 dendritic cells. Proteomics. 2008. 8:2384–2393.
Article10. Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001. 31:1287–1312.
Article11. Fyhrquist-Vanni N, Alenius H, Lauerma A. Contact dermatitis. Dermatol Clin. 2007. 25:613–623.
Article12. Banchereau J, Steinmann RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article13. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.
Article14. Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J Immunol. 2008. 180:3057–3064.
Article15. Grassi F, Dezutter-Dambuyant C, McIlroy D, Jacquet C, Yoneda K, Imamura S, et al. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J Leukoc Biol. 1998. 64:484–493.
Article16. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994. 179:1109–1118.
Article17. Choi GS, Kang JM, Lee MG. Analysis of methods for the generation of dendritic cells from human peripheral blood monocytes. Yonsei Med J. 2000. 41:642–650.
Article18. Kim DS, Kim DH, Byamba D, Lee TH, Cho YH, Lee MG. The production and functions of reactive oxygen species in mouse bone marrow-derived dendritic cells by various haptens and irritants. Korean J Dermatol. 2008. 46:1470–1477.
Article19. Mizuashi M, Ohtani T, Nakagawa S, Aiba S. Redox imbalance induced by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 2005. 124:579–586.
Article20. Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999. 26:232–238.
Article21. Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003. 65:1575–1582.
Article22. Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007. 43:995–1022.
Article23. Armstrong JS, Whiteman M. Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol. 2007. 80:355–377.
Article24. Yoshida Y, Shimakawa S, Itoh N, Niki E. Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radic Res. 2003. 37:861–872.
Article25. Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, Castro-Obregón S. Function of reactive oxygen species during animal development: passive or active? Dev Biol. 2008. 320:1–11.26. Papa L, Gomes E, Rockwell P. Reactive oxygen species induced by proteasome inhibition in neuronal cells mediate mitochondrial dysfunction and a caspase-independent cell death. Apoptosis. 2007. 12:1389–1405.27. Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH, Chung JH. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic Biol Med. 2008. 44:635–645.
Article28. Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003. 9:169–176.
Article29. Arouma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989. 6:593–597.
Article30. Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002. 265:49–72.
Article31. Stepnik M, Arkusz J. Molecular events associated with dendritic cells activation by contact sensitizers. Int J Occup Med Environ Health. 2003. 16:191–199.
Article32. Aiba S, Manome H, Nakagawa S, Mollah ZU, Mizuashi M, Ohtani T, et al. p38 Mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J Invest Dermatol. 2003. 120:390–399.
Article33. Trompezinski S, Migdal C, Tailhardat M, Le Varlet B, Courtellemont P, Haftek M, et al. Characterization of early events involved in human dendritic cell maturation induced by sensitizers: cross talk between MAPK signalling pathways. Toxicol Appl Pharmacol. 2008. 230:397–406.
Article34. Watanabe H, Gaide O, Pétrilli V, Martinon F, Contassot E, Roques S, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007. 127:1956–1963.
Article35. Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003. 171:3010–3018.
Article36. Na K, Kim KE, Park ST, Kim TY. EC-SOD suppresses contact hypersensitivity in mouse skin by impairing Langerhans cell migration. J Invest Dermatol. 2007. 127:1930–1937.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Production and Functions of Reactive Oxygen Species in Mouse Bone Marrow-derived Dendritic Cells by Various Haptens and Irritants
- Effect of Salicylate on the Monocyte Chemoattractant Protein-1 Expression and Intracellular Reactive Oxygen Species Formation in Human Mesangial Cells
- A Case of Epoxy Resin-induced Airborne Allergic Contact Dermatitis
- Keratinocytes-Derived Reactive Oxygen Species Play an Active Role to Induce Type 2 Inflammation of the Skin: A Pathogenic Role of Reactive Oxygen Species at the Early Phase of Atopic Dermatitis
- Do Reactive Oxygen Species Cause Aging?