Allergy Asthma Immunol Res.
2013 Nov;5(6):389-396. 10.4168/aair.2013.5.6.389.
MRI Reveals Edema in Larynx (But Not in Brain) During Anaphylactic Hypotension in Anesthetized Rats
- Affiliations
-
- 1Department of Radiology, Kanazawa Medical University, Uchinada, Japan.
- 2Department of Physiology II, Kanazawa Medical University, Uchinada, Japan. shibamo@kanazawa-med.ac.jp
- 3Department of Colorectal Surgery, The Fourth Affiliated Hospital of China Medical University, Shenyang, China.
- KMID: 2260283
- DOI: http://doi.org/10.4168/aair.2013.5.6.389
Abstract
- PURPOSE
Anaphylactic shock is sometimes accompanied by local interstitial edema due to increased vascular permeability. We performed magnetic resonance imaging (MRI) to compare edema in the larynx and brain of anesthetized rats during anaphylactic hypotension versus vasodilator-induced hypotension.
METHODS
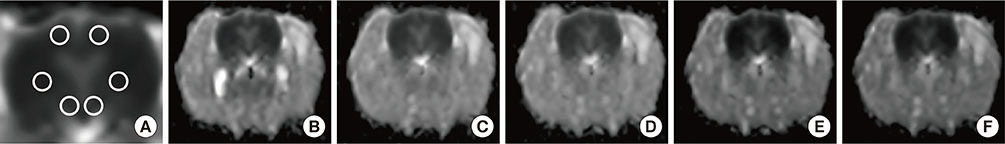
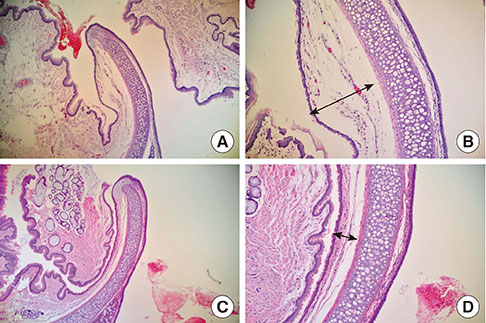
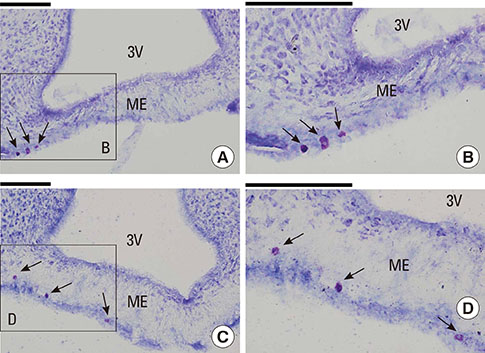
Male Sprague Dawley rats were subjected to hypotension induced by the ovalbumin antigen (n=7) or a vasodilator sodium nitroprusside (SNP; n=7). Apparent diffusion coefficient (ADC) and T2-relaxation time (T2RT) were quantified on MRI performed repeatedly for up to 68 min after the injection of either agent. The presence of laryngeal edema was also examined by histological examination. Separately, the occurrence of brain edema was assessed by measuring brain water content using the wet/dry method in rats with anaphylaxis (n=5) or SNP (n=5) and the non-hypotensive control rats (n=5). Mast cells in hypothalamus were morphologically examined.
RESULTS
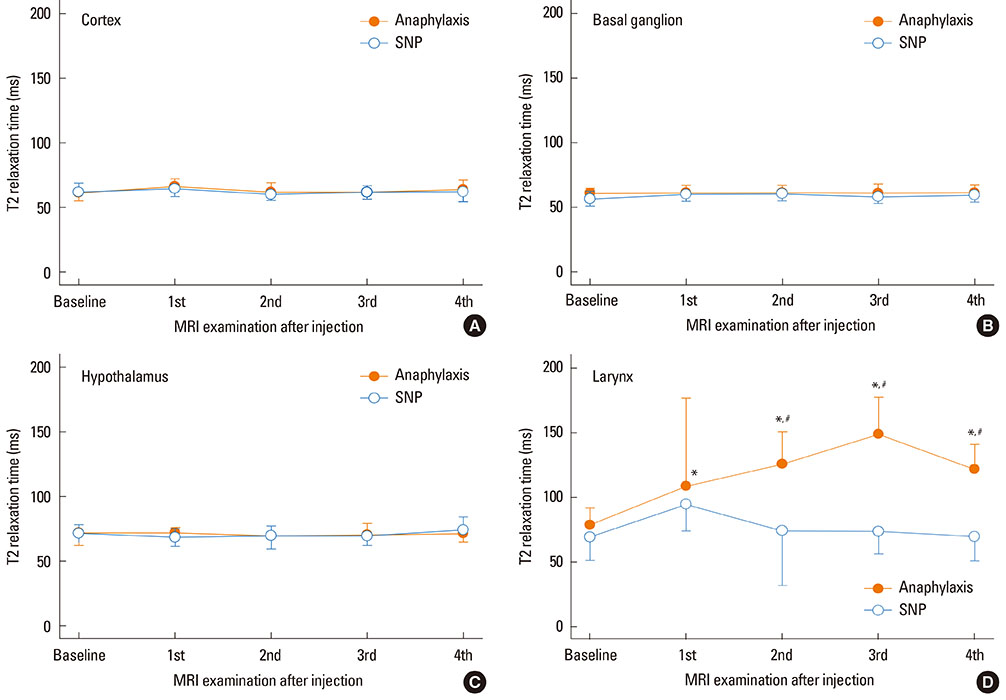
Mean arterial blood pressure similarly decreased to 35 mmHg after an injection of the antigen or SNP. Hyperintensity on T2-weighted images (as reflected by elevated T2RT) was found in the larynx as early as 13 min after an injection of the antigen, but not SNP. A postmortem histological examination revealed epiglottic edema in the rats with anaphylaxis, but not SNP. In contrast, no significant changes in T2RT or ADC were detectable in the brains of any rats studied. In separate experiments, the quantified brain water content did not increase in either anaphylaxis or SNP rats, as compared with the non-hypotensive control rats. The numbers of mast cells with metachromatic granules in the hypothalamus were not different between rats with anaphylaxis and SNP, suggesting the absence of anaphylactic reaction in hypothalamus.
CONCLUSION
Edema was detected using the MRI technique in the larynx during rat anaphylaxis, but not in the brain.
Keyword
MeSH Terms
Figure
Reference
-
1. Brown SG. The pathophysiology of shock in anaphylaxis. Immunol Allergy Clin North Am. 2007; 27:165–175.2. Fisher MM. Clinical observations on the pathophysiology and treatment of anaphylactic cardiovascular collapse. Anaesth Intensive Care. 1986; 14:17–21.3. Shin CH, Lee YH, Kim YM, Park SH, Sung IY, Choi SW, Park SE. Severe oropharyngeal angioedema caused by propofol: a case report. Korean J Anesthesiol. 2006; 50:S68–S70.4. You BC, Jang AS, Han JS, Cheon HW, Park JS, Lee JH, Park SW, Kim DJ, Park CS. A case of propofol-induced oropharyngeal angioedema and bronchospasm. Allergy Asthma Immunol Res. 2012; 4:46–48.5. Greenberger PA, Ditto AM. Chapter 24: anaphylaxis. Allergy Asthma Proc. 2012; 33:Suppl 1. S80–S83.6. Sampson HA, Muñoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, Decker WW, Furlong TJ, Galli SJ, Golden DB, Gruchalla RS, Harlor AD Jr, Hepner DL, Howarth M, Kaplan AP, Levy JH, Lewis LM, Lieberman PL, Metcalfe DD, Murphy R, Pollart SM, Pumphrey RS, Rosenwasser LJ, Simons FE, Wood JP, Camargo CA Jr. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005; 115:584–591.7. Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010; 125:S161–S181.8. Klatzo I. Presidential address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967; 26:1–14.9. Katzman R, Clasen R, Klatzo I, Meyer JS, Pappius HM, Waltz AG. Report of Joint Committee for Stroke Resources. IV. Brain edema in stroke. Stroke. 1977; 8:512–540.10. Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat. 2002; 200:639–646.11. Esen F, Erdem T, Aktan D, Orhan M, Kaya M, Eraksoy H, Cakar N, Telci L. Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit Care. 2005; 9:R18–R23.12. Loubinoux I, Volk A, Borredon J, Guirimand S, Tiffon B, Seylaz J, Méric P. Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke. 1997; 28:419–426.13. Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990; 14:330–346.14. Shibamoto T, Cui S, Ruan Z, Liu W, Takano H, Kurata Y. Hepatic venoconstriction is involved in anaphylactic hypotension in rats. Am J Physiol Heart Circ Physiol. 2005; 289:H1436–H1441.15. Xu F, Yu ZY, Ding L, Zheng SY. Experimental studies of erythropoietin protection following traumatic brain injury in rats. Exp Ther Med. 2012; 4:977–982.16. Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, Nagao S, Xi G, Keep RF, Itano T. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008; 39:463–469.17. Wahl M, Schilling L. Regulation of cerebral blood flow--a brief review. Acta Neurochir Suppl (Wien). 1993; 59:3–10.18. Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010; 30:689–702.19. Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995; 136:5745–5750.20. Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984; 81:2572–2576.21. Matsumoto I, Inoue Y, Shimada T, Aikawa T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J Exp Med. 2001; 194:71–78.22. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896; 19:312–326.23. Gerriets T, Stolz E, Walberer M, Müller C, Kluge A, Kaps M, Fisher M, Bachmann G. Middle cerebral artery occlusion during MR-imaging: investigation of the hyperacute phase of stroke using a new in-bore occlusion model in rats. Brain Res Brain Res Protoc. 2004; 12:137–143.24. Lefèbvre PR, Cordonnier M, Balériaux D, Chamart D. An unusual cause of visual loss: involvement of bilateral lateral geniculate bodies. AJNR Am J Neuroradiol. 2004; 25:1544–1548.25. Yucel N, Kayaalp C, Liceli A, Baysal T, Yilmaz M. Blindness following rupture of hepatic hydatid cyst: a case report. Adv Med Sci. 2009; 54:299–301.26. Schäbitz WR, Berger C, Knauth M, Meinck HM, Steiner T. Hypoxic brain damage after intramuscular self-injection of diclofenac for acute back pain. Eur J Anaesthesiol. 2001; 18:763–765.27. Hoffmann J, Erb K, Klingebiel R, Siebert E. Hypoxic brain injury sparing the posterior circulation. Neurology. 2010; 74:1476.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Ketamine Anesthesia on the Formation of Brain Edema During Focal Ischemia in Rats
- Anaphylactic Reaction after Aprotinin Reexposure: A case report
- Spontaneous Intracranial Hypotension: MRI findings
- Effect of Preservation of Head Temperature on the Formation of Brain Edema during Focal Ischemia in Rats
- Spinal dural enhancement in spontaneous intracranial hypotension on MRI