Korean J Hematol.
2007 Mar;42(1):24-32. 10.5045/kjh.2007.42.1.24.
Role of Pertussis Toxin-sensitive G Protein-coupled Receptor Signaling in the Proliferation of Bone Marrow Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Chungnam National University, Daejon, Korea. deogyeon@cnu.ac.kr
- KMID: 2252265
- DOI: http://doi.org/10.5045/kjh.2007.42.1.24
Abstract
-
BACKGROUND: Bone marrow (BM) mesenchymal stem cells (MSCs) can be expanded over 20~30 cell doublings in vitro even in the absence of any growth factors. However, the mechanisms that govern MSC proliferation are not well understood.
METHODS
We investigated the role of signaling of the pertussis toxin (PTX)-sensitive G protein-coupled receptor in the proliferation of BM MSCs.
RESULTS
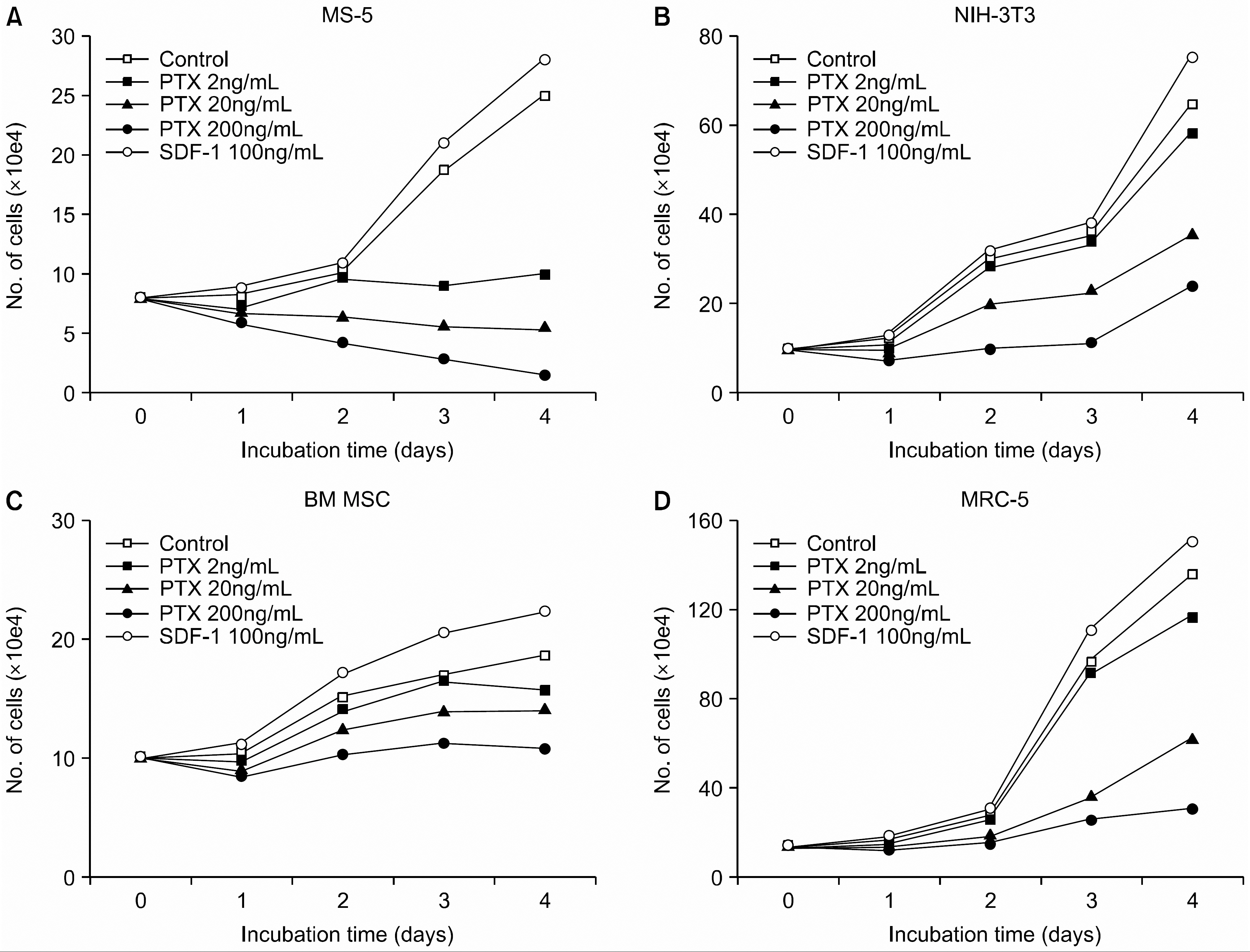
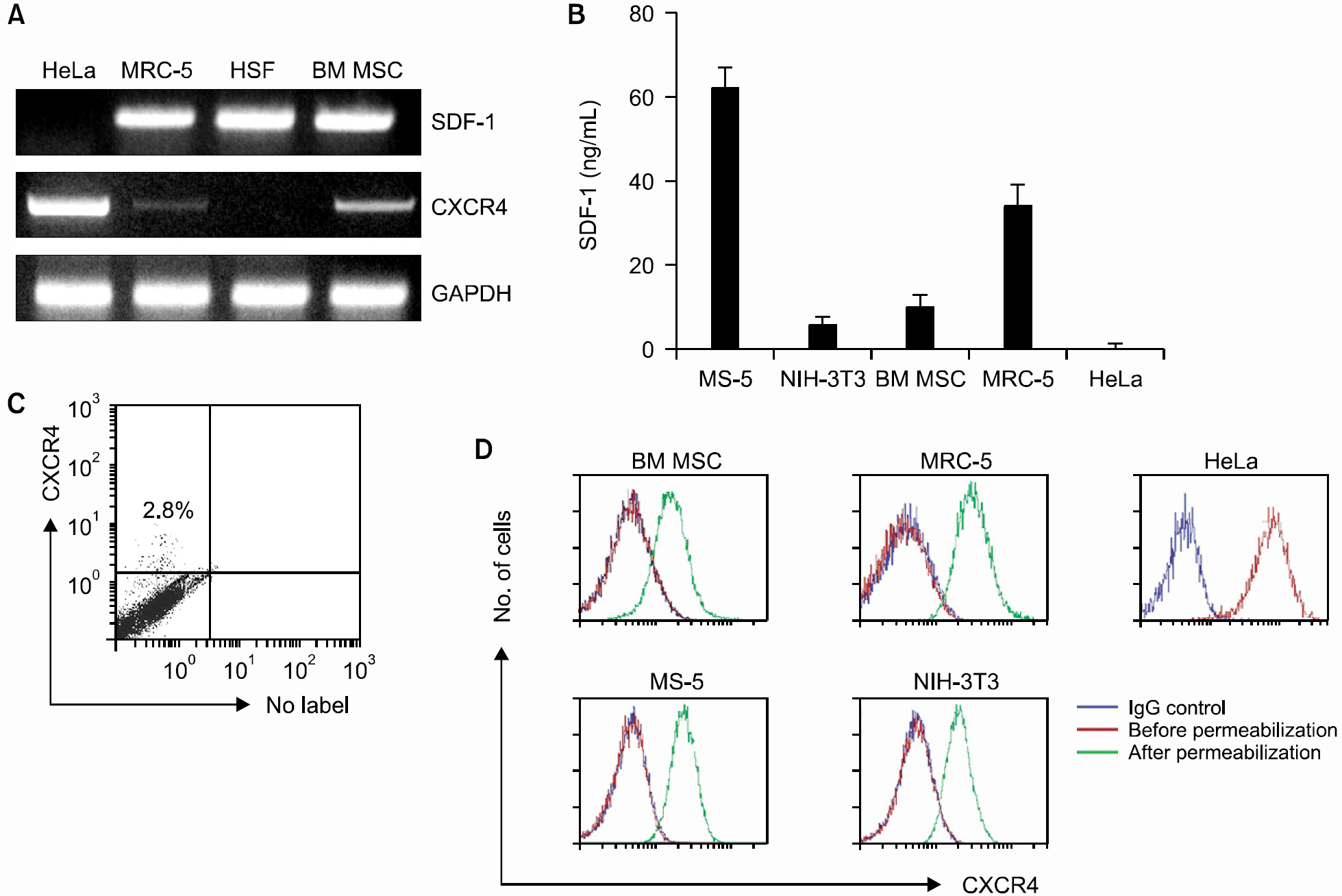
PTX inhibited the proliferation of human BM MSCs and murine BM stromal MS-5 cells in a dose-dependent manner. Among the chemokines produced by the BM stromal cells, stromal cell-derived factor-1 (SDF-1) enhanced the proliferation of BM MSCs, while MIP-1alpha, MCP-3 or RANTES did not. PTX also inhibited the proliferation of some fibroblasts, such as MRC-5 and NIH-3T3, but did not affect the proliferation of HeLa and HSF cells. HSF cells did not express CXCR4 mRNA, but did produce SDF-1. In contrast, HeLa cells expressed CXCR4 strongly on the cell surface, but did not produce SDF-1. BM MSCs, MS-5, MRC-5, and NIH-3T3 cells all expressed CXCR4 minimally on the cell surface. These cells, however, had abundant CXCR4 protein in their cytoplasm, which was demonstrated by flow cytometric analysis performed after permeabilization of the cells. In addition, an ELISA performed on the culture supernatants of the cells revealed that these cells constitutively produce and secrete SDF-1.
CONCLUSION
These results indicate that the signaling through the PTX-sensitive G protein-coupled receptor, which is induced by autocrine factors, plays an important role in the proliferation of BM MSCs and in some fibroblasts, and that SDF-1 is the most probable candidate for the autocrine growth factor.
Keyword
MeSH Terms
-
Bone Marrow*
Cell Proliferation
Chemokine CCL3
Chemokine CCL5
Chemokines
Cytoplasm
Enzyme-Linked Immunosorbent Assay
Fibroblasts
HeLa Cells
Humans
Intercellular Signaling Peptides and Proteins
Mesenchymal Stromal Cells*
NIH 3T3 Cells
Pertussis Toxin
RNA, Messenger
Stromal Cells
Whooping Cough*
Chemokine CCL3
Chemokine CCL5
Chemokines
Intercellular Signaling Peptides and Proteins
Pertussis Toxin
RNA, Messenger
Figure
Cited by 1 articles
-
Bortezomib inhibits the survival and proliferation of bone marrow stromal cells
Ha-Yon Kim, Ji-Young Moon, Haewon Ryu, Yoon-Seok Choi, Ik-Chan Song, Hyo-Jin Lee, Hwan-Jung Yun, Samyong Kim, Deog-Yeon Jo
Blood Res. 2015;50(2):87-96. doi: 10.5045/br.2015.50.2.87.
Reference
-
1). Javazon EH., Beggs KJ., Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004. 32:414–25.
Article2). Kortesidis A., Zannettino A., Isenmann S., Shi S., La-pidot T., Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005. 105:3793–801.
Article3). Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek AM., Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006. 24:1030–41.
Article4). Sordi V., Malosio ML., Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005. 106:419–27.
Article5). Psenak O., Sefc L., Sykora V., Chang KT., Necas E. Cytokine gene expression in regenerating haematopoietic tissues of mice after cyclophosphamide treatment. Acta Haematol. 2003. 109:68–75.
Article6). Bromley SK., Peterson DA., Gunn MD., Dustin ML. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J Immunol. 2000. 165:15–9.
Article7). Gentilini G., Kirschbaum NE., Augustine JA., Aster RH., Visentin GP. Inhibition of human umbilical vein endothelial cell proliferation by the CXC chemokine, platelet factor 4 (PF4), is associated with impaired downregulation of p21 (Cip1/WAF1). Blood. 1999. 93:25–33.8). Luster AD., Greenberg SM., Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995. 182:219–31.
Article9). Sanchez X., Suetomi K., Cousins-Hodges B., Horton JK., Navarro J. CXC chemokines suppress proliferation of myeloid progenitor cells by activation of the CXC chemokine receptor 2. J Immunol. 1998. 160:906–10.10). Huang Z. Structure, function and modulation of chemokine receptors: members of the g-protein-coupled receptor superfamily. Mini Rev Med Chem. 2002. 2:373–83.
Article11). Reisine T. Pertussis toxin in the analysis of receptor mechanisms. Biochem Pharmacol. 1990. 39:1499–504.
Article12). Kaslow HR., Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 1992. 6:2684–90.
Article13). Nishi N., Ishikawa R., Inoue H, et al. In vitro longterm culture of human primitive hematopoietic cells supported by murine stromal cell line MS-5. Leukemia. 1997. 11(Suppl 3):468–73.14). Kim CH., Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998. 91:100–10.
Article15). Rickard DJ., Kassem M., Hefferan TE., Sarkar G., Spel-sberg TC., Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996. 11:312–24.
Article16). Stenderup K., Justesen J., Clausen C., Kassem M. A-ging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003. 33:919–26.
Article17). Reyes M., Lund T., Lenvik T., Aguiar D., Koodie L., Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001. 98:2615–25.
Article18). Lodie TA., Blickarz CE., Devarakonda TJ, et al. Systematic analysis of reportedly distinct populations of multipotent bone marrow-derived stem cells reveals a lack of distinction. Tissue Eng. 2002. 8:739–51.
Article19). Bonig H., Rohmer L., Papayannopoulou T. Longterm functional impairment of hemopoietic progenitor cells engineered to express the S1 catalytic subunit of pertussis toxin. Exp Hematol. 2005. 33:689–98.
Article20). He YX., Hewlett E., Temeles D., Quesenberry P. Inhibition of interleukin 3 and colony-stimulating factor 1-stimulated marrow cell proliferation by pertussis toxin. Blood. 1988. 71:1187–95.
Article21). Papayannopoulou T., Priestley GV., Bonig H., Naka-moto B. The role of G-protein signaling in hematopoietic stem/progenitor cell mobilization. Blood. 2003. 101:4739–47.
Article22). Hinds PW 2nd., Yin C., Salvato MS., Pauza CD. Pertussis toxin induces lymphocytosis in rhesus macaques. J Med Primatol. 1996. 25:375–81.
Article23). Neel NF., Schutyser E., Sai J., Fan GH., Richmond A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005. 16:637–58.
Article24). Juarez J., Bendall L., Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des. 2004. 10:1245–59.25). Lee Y., Gotoh A., Kwon HJ, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002. 99:4307–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stromal Cell-Derived Factor-1 Promotes Myeloma Cell Growth in Both Autocrine and Paracrine Manners
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Analysis of a Sphingosine 1-phosphate Receptor hS1P3 in Rat Hepatoma Cells
- Role of Angiogenic Stem Cells in Bone Regeneration
- Native Low-Density Lipoprotein-Dependent Interleukin-8 Production Through Pertussis Toxin-Sensitive G-Protein Coupled Receptors and Hydrogen Peroxide Generation Contributes to Migration of Human Aortic Smooth Muscle Cells