Korean J Hematol.
2008 Sep;43(3):127-137. 10.5045/kjh.2008.43.3.127.
Stromal Cell-Derived Factor-1 Promotes Myeloma Cell Growth in Both Autocrine and Paracrine Manners
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Internal Medicine, College of Medicine, Chungnam National University, Daejeon, Korea. deogyeon@cnu.ac.kr
- KMID: 2252178
- DOI: http://doi.org/10.5045/kjh.2008.43.3.127
Abstract
-
BACKGROUND: The question as to whether stromal cell-derived factor-1 (SDF-1) stimulated myeloma cell growth has been controversial. We explored the possibility that SDF-1 may function as an autocrine growth factor of myeloma cells.
METHODS
CD138+ primary bone marrow myeloma cells and myeloma cell lines (RPMI8226, U266, and ARH77) were used. Chemotaxis in response to SDF-1 of the cells was analyzed using Transwells(TM). Cell proliferation was measured by a colorimetric assay. SDF-1 mRNA expression was analyzed by RT-PCR (reverse-transcription-polymerase chain reaction). SDF-1 and interleukin-6 (IL-6) receptor expression as well as signaling molecule phosphorylation levels, were examined using Western blot analysis. Concentrations of SDF-1 in the cell culture supernatants were measured by ELISA assay.
RESULTS
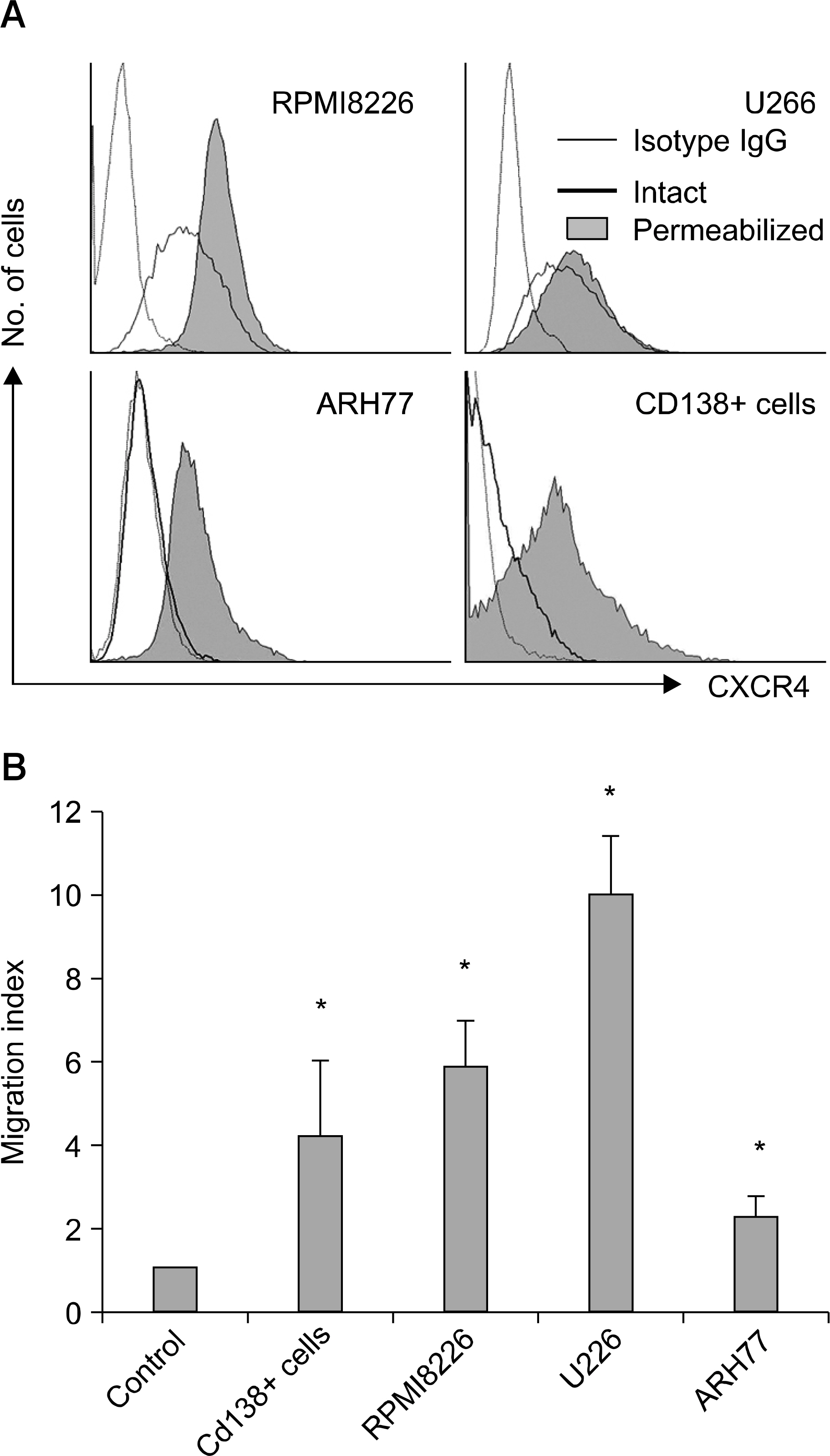
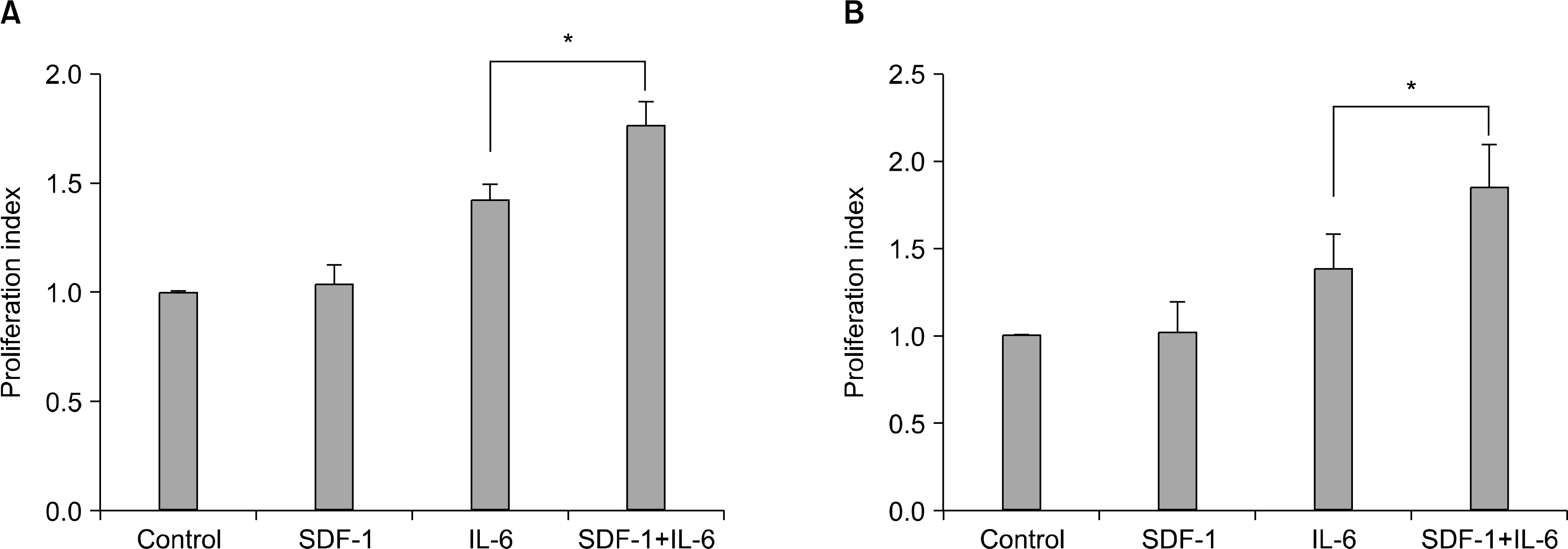
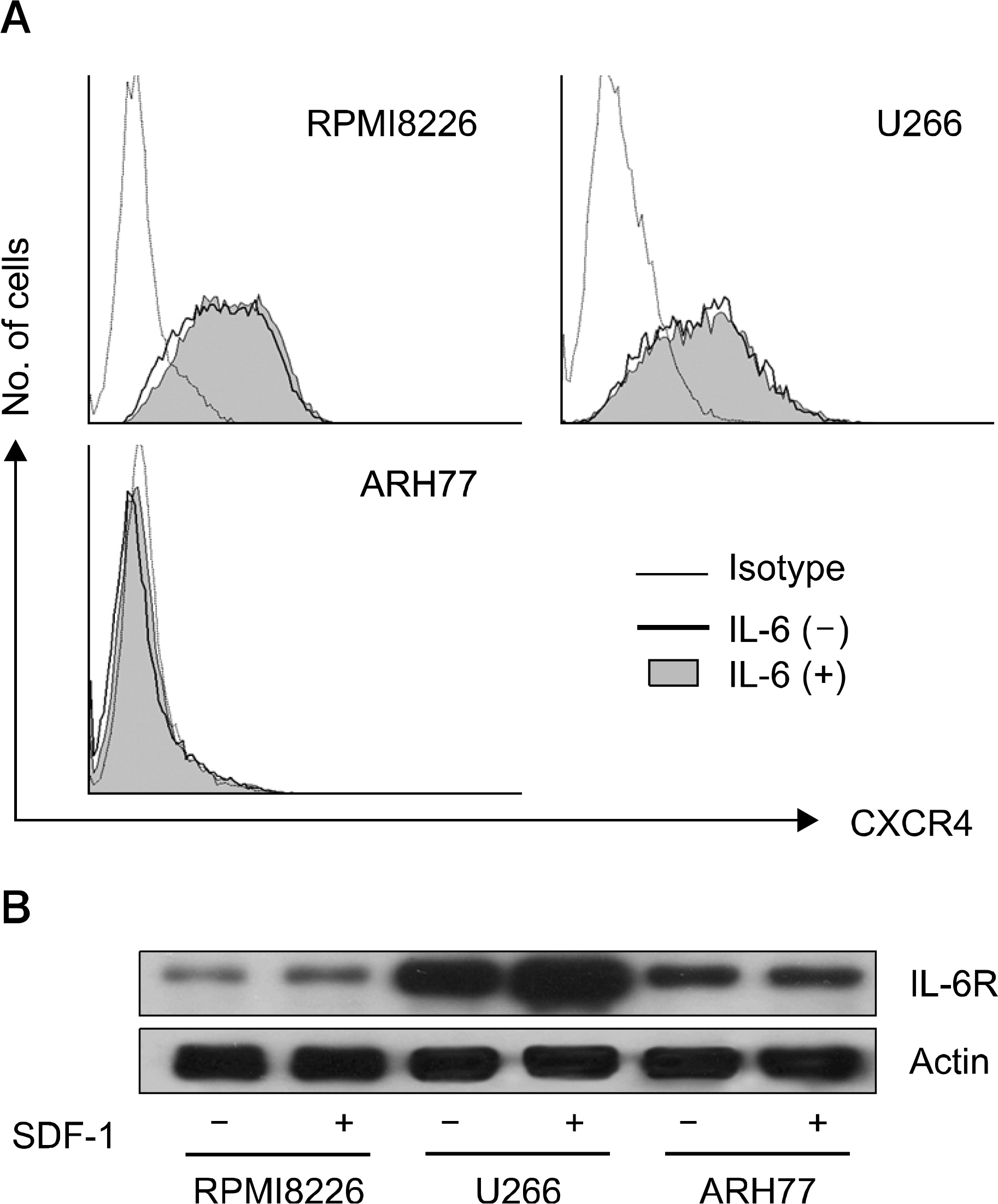
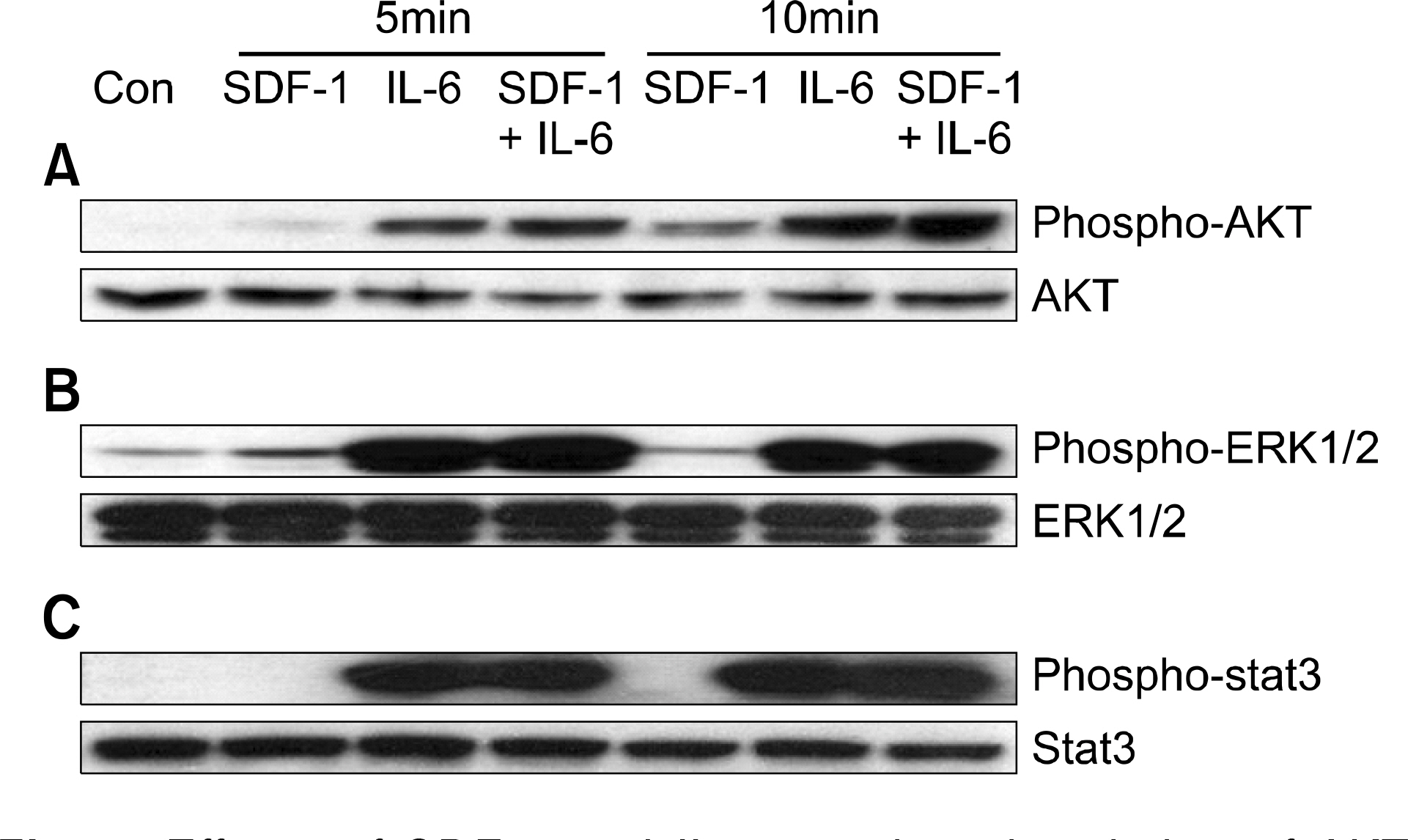
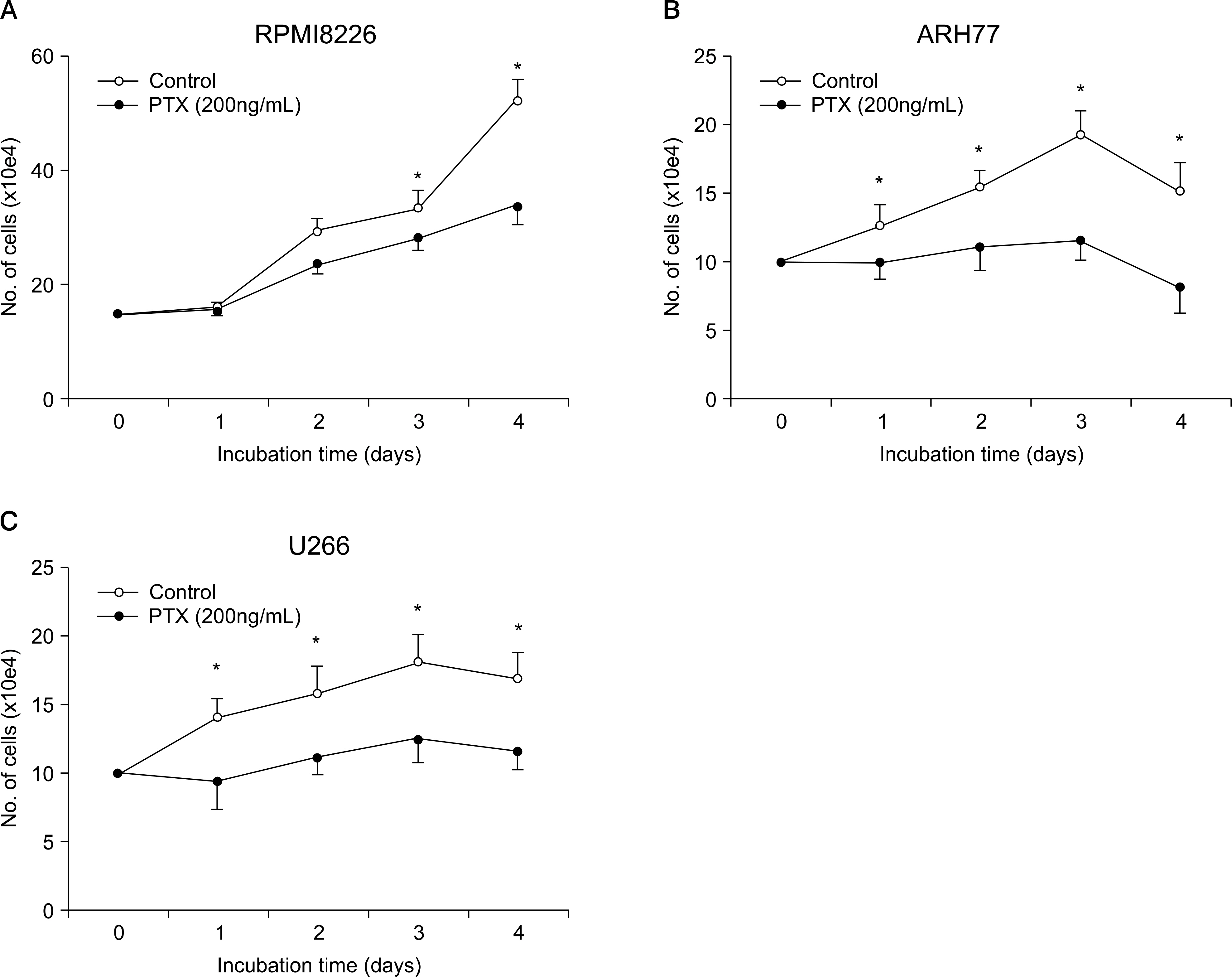
SDF-1 alone had no discernible effect on the proliferation of CD138+ primary myeloma cells or myeloma cell lines. In contrast, SDF-1 significantly enhanced IL-6-induced proliferation of these cells. SDF-1 up-regulated the expression of IL-6 receptor and enhanced phosphorylation of AKT in an additive manner with IL-6. Co-culture of the myeloma cells with umbilical vein endothelial cells over-expressing the SDF-1 gene revealed that SDF-1 played an important role in not only the migration of the cells underneath the stromal cells but also the proliferation of the cells in contact with stromal cells. All the myeloma cell lines expressed SDF-1 mRNA, and SDF-1 was detected in the culture supernatants of the cells. The G protein-coupled receptor inhibitor, pertussis toxin, inhibited the proliferation of these cells in suspension cultures.
CONCLUSION
SDF-1, most likely in concert with IL-6, enhanced the proliferation of myeloma cells in a both paracrine and autocrine manner.
Keyword
MeSH Terms
-
Blotting, Western
Bone Marrow
Cell Culture Techniques
Cell Line
Cell Proliferation
Chemotaxis
Coculture Techniques
Endothelial Cells
Enzyme-Linked Immunosorbent Assay
Interleukin-6
Mesenchymal Stromal Cells
Multiple Myeloma
Pertussis Toxin
Phosphorylation
Receptors, Interleukin-6
RNA, Messenger
Stromal Cells
Umbilical Veins
Interleukin-6
Pertussis Toxin
RNA, Messenger
Receptors, Interleukin-6
Figure
Reference
-
1). Peled A., Petit I., Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999. 283:845–8.
Article2). Aiuti A., Webb IJ., Bleul C., Springer T., Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997. 185:111–20.
Article3). Dürig J., Schmücker U., Dührsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001. 15:752–6.
Article4). Sanz-Rodriguez F., Hidalgo A., Teixidó J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001. 97:346–51.5). Möller C., Strömberg T., Juremalm M., Nilsson K., Nilsson G. Expression and function of chemokine receptors in human multiple myeloma. Leukemia. 2003. 17:203–10.
Article6). Parmo-Cabañas M., Bartolomé RA., Wright N., Hidalgo A., Drager AM., Teixidó J. Integrin alpha4beta1 involvement in stromal cell-derived factor-1alpha-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res. 2004. 294:571–80.7). Zannettino AC., Farrugia AN., Kortesidis A, et al. Elevated serum levels of stromal-derived factor-1al-pha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005. 65:1700–9.8). Pellegrino A., Ria R., Di Pietro G, et al. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br J Haematol. 2005. 129:248–56.
Article9). Guleng B., Tateishi K., Ohta M, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005. 65:5864–71.
Article10). Hideshima T., Chauhan D., Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002. 1:539–44.11). Jaffe EA., Nachman RL., Becker CG., Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973. 52:2745–56.
Article12). Ramalingam R., Rafii S., Worgall S., Brough DE., Crystal RG. E1(−)E4(+) adenoviral gene transfer vectors function as a "pro-life" signal to promote survival of primary human endothelial cells. Blood. 1999. 93:2936–44.
Article13). Aikawa S., Hatta Y., Tanaka M, et al. Requirement of soluble factors produced by bone marrow stromal cells on the growth of novel established human myeloma cell line. Int J Oncol. 2003. 22:631–7.14). Derksen PW., de Gorter DJ., Meijer HP, et al. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia. 2003. 17:764–74.
Article15). Qiang YW., Yao L., Tosato G., Rudikoff S. Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood. 2004. 103:301–8.
Article16). Caligaris-Cappio F., Gregoretti MG., Merico F, et al. Bone marrow microenvironment and the progression of multiple myeloma. Leuk Lymphoma. 1992. 8:15–22.
Article17). Nefedova Y., Landowski TH., Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003. 17:1175–82.
Article18). Uchiyama H., Barut BA., Mohrbacher AF., Chauhan D., Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993. 82:3712–20.
Article19). Le Gouill S., Podar K., Amiot M, et al. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood. 2004. 104:2886–92.
Article20). Chatterjee M., Hönemann D., Lentzsch S, et al. In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood. 2002. 100:3311–8.
Article21). Michigami T., Shimizu N., Williams PJ, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000. 96:1953–60.22). Neben S., Anklesaria P., Greenberger J., Mauch P. Quantitation of murine hematopoietic stem cells in vitro by limiting dilution analysis of cobblestone area formation on a clonal stromal cell line. Exp Hematol. 1993. 21:438–43.23). Breems DA., Blokland EA., Neben S., Ploemacher RE. Frequency analysis of human primitive haematopoietic stem cell subsets using a cobblestone area forming cell assay. Leukemia. 1994. 8:1095–104.24). Lataillade JJ., Clay D., Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000. 95:756–68.
Article25). Lee Y., Gotoh A., Kwon HJ, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002. 99:4307–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigallocatechin-3-gallate inhibits paracrine and autocrine hepatocyte growth factor/scatter factor-induced tumor cell migration and invasion
- Bortezomib inhibits the survival and proliferation of bone marrow stromal cells
- Correlations amongst Insulin-like Growth Factor-I, Insulin-like Growth Factor Binding Protein-3, Growth Hormone and Estradiol in Follicular Fluid of the Patients with Ovulation Induction
- The Effect of Vascular Endothelial Growth Factor and basic Fibroblast Growth Factor on the Growth of Endometrial Stromal Sarcoma
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation