Yonsei Med J.
2011 May;52(3):413-419. 10.3349/ymj.2011.52.3.413.
Native Low-Density Lipoprotein-Dependent Interleukin-8 Production Through Pertussis Toxin-Sensitive G-Protein Coupled Receptors and Hydrogen Peroxide Generation Contributes to Migration of Human Aortic Smooth Muscle Cells
- Affiliations
-
- 1Department of Biology, Kangwon National University, Chuncheon, Korea. ryoosw08@kangwon.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1777012
- DOI: http://doi.org/10.3349/ymj.2011.52.3.413
Abstract
- PURPOSE
Stimulation of human aortic smooth muscle cells (hAoSMCs) with native low-density lipoprotein (nLDL) induced the production of interleukin-8 (IL-8) that is involved in the pathogenesis of cardiovascular diseases. However, the process of signal transduction of nLDL was currently uncharacterized. Therefore, the aim of this study was to investigate the signal transduction pathway of nLDL-dependent IL-8 production and the effect of IL-8 on hAoSMCs migration.
MATERIALS AND METHODS
nLDL was prepared by ultracentrifugation with density-adjusted human serum of normocholesterolemia. In hAoSMCs, IL-8 secreted to medium was measured using ELISA assay, and Western blot analysis was performed to detect p38 MAPK activation as a key regulator of IL-8 production. nLDL-dependent H2O2 generation was determined by microscopic analysis using 2',7'-dichlorofluoroscein diacetate (DCF-DA). IL-8-induced migration of hAoSMCs was evaluated by counting the cell numbers moved to lower chamber using Transwell plates.
RESULTS
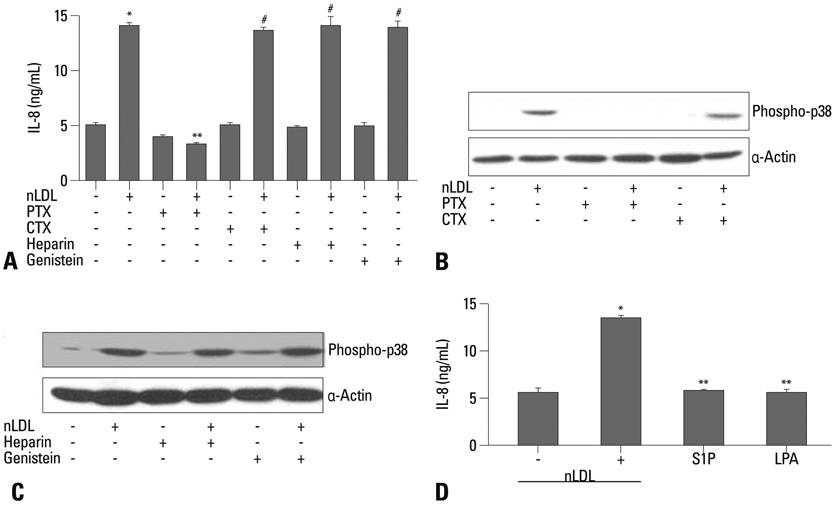
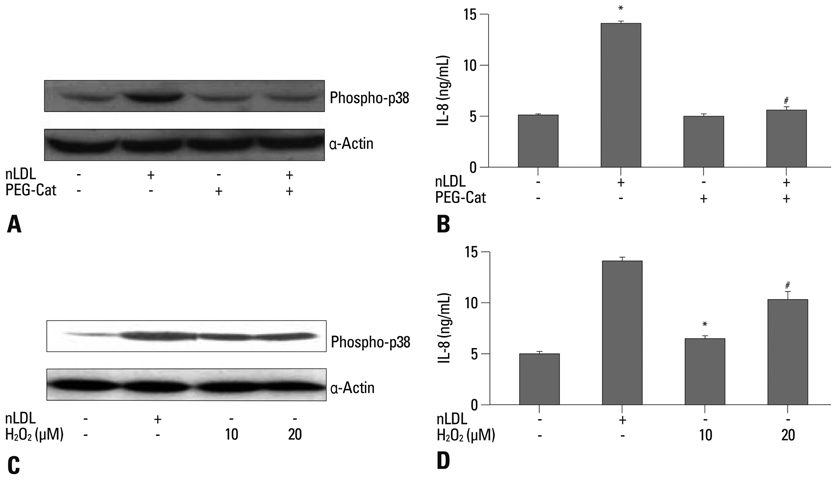
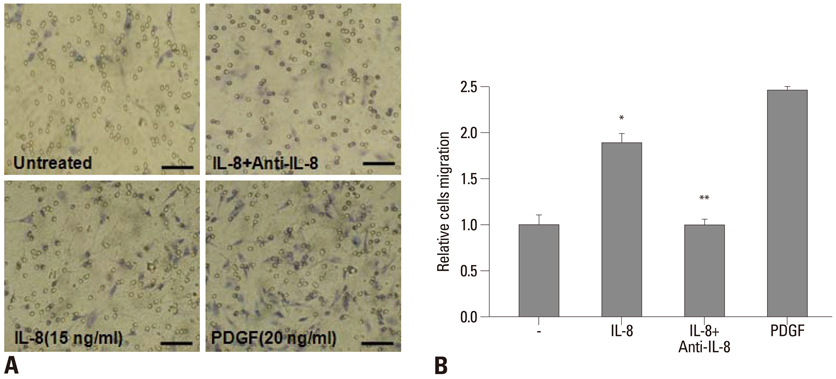
nLDL-induced IL-8 production was completely blocked by preincubation of hAoSMCs with pertussis toxin (PTX), which inhibited nLDL-dependent p38 MAPK phosphorylation. PTX-sensitive G-protein coupled receptor was responsible for nLDL-dependent H2O2 generation that was abrogated with preincubation of the cells with of polyethylene glycol-conjugated catalase (PEG-Cat). Pretreatment of PEG-Cat prevented nLDL-induced p38 MAPK phosphorylation and IL-8 production, which was partly mimicked by treatment with exogenous H2O2. Finally, IL-8 increased hAoSMCs migration that was completely blocked by incubation with IL-8 neutralizing antibody.
CONCLUSION
PTX-sensitive G-protein coupled receptor-dependent H2O2 generation by nLDL plays a critical role in IL-8 production in hAoSMC, and IL-8 may contribute to atherogenesis through increased migration of hAoSMCs.
Keyword
MeSH Terms
-
Cell Movement/*physiology
Cells, Cultured
Humans
Hydrogen Peroxide/*metabolism
Interleukin-8/*biosynthesis
Lipoproteins, LDL/*pharmacology
Muscle, Smooth, Vascular/cytology/*metabolism
Myocytes, Smooth Muscle/cytology/*metabolism
Pertussis Toxin/pharmacology
Phosphorylation/drug effects
Reactive Oxygen Species/metabolism
Receptors, G-Protein-Coupled/*physiology
Signal Transduction
p38 Mitogen-Activated Protein Kinases/metabolism
Figure
Reference
-
1. Grundy SM. Cholesterol and coronary heart disease. Future directions. JAMA. 1990. 264:3053–3059.
Article2. Steinberg D, Witztum JL. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990. 264:3047–3052.
Article3. Vega GL, Grundy SM. Primary hypertriglyceridemia with borderline high cholesterol and elevated apolipoprotein B concentrations. Comparison of gemfibrozil vs lovastatin therapy. JAMA. 1990. 264:2759–2763.
Article4. Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000. 33:Suppl. S85–S97.5. Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981. 103:181–190.6. Stary HC, Blankenhorn DH, Chandler AB, Glogov S, Insull W Jr, Richardson M, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1992. 12:120–134.
Article7. Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989. 9:I19–I32.8. Rosenfeld ME, Tsukada T, Gown AM, Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987. 7:9–23.
Article9. Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006. 114:1531–1544.
Article10. Heo KS, Kim DU, Ryoo S, Nam M, Baek ST, Kim L, et al. PPARgamma activation abolishes LDL-induced proliferation of human aortic smooth muscle cells via SOD-mediated down-regulation of superoxide. Biochem Biophys Res Commun. 2007. 359:1017–1023.
Article11. Lee HS, Son SM, Kim YK, Hong KW, Kim CD. NAD(P)H oxidase participates in the signaling events in high glucose-induced proliferation of vascular smooth muscle cells. Life Sci. 2003. 72:2719–2730.
Article12. Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001. 276:21938–21942.
Article13. Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999. 398:718–723.
Article14. Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998. 101:353–363.
Article15. Yue TL, Wang X, Sung CP, Olson B, Mckenna PJ, Gu JL, et al. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994. 75:1–7.
Article16. Ryoo SW, Kim DU, Won M, Chung KS, Jang YS, Oh GT, et al. Native LDL induces interleukin-8 expression via H2O2, p38 Kinase, and activator protein-1 in human aortic smooth muscle cells. Cardiovasc Res. 2004. 62:185–193.
Article17. Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, et al. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003. 108:1746–1752.
Article18. Maupas-Schwalm F, Augé N, Robinet C, Cambus JP, Parsons SJ, Salvayre R, et al. The sphingomyelin/ceramide pathway is involved in ERK1/2 phosphorylation, cell proliferation, and uPAR overexpression induced by tissue-type plasminogen activator. FASEB J. 2004. 18:1398–1400.
Article19. Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998. 273:15022–15029.
Article20. Heo KS, Kim DU, Kim L, Nam M, Baek ST, Park SK, et al. Activation of PKCbeta(II) and PKCtheta is essential for LDL-induced cell proliferation of human aortic smooth muscle cells via Gi-mediated Erk1/2 activation and Egr-1 upregulation. Biochem Biophys Res Commun. 2008. 368:126–131.
Article21. Baas AS, Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2- in vascular smooth muscle cells. Circ Res. 1995. 77:29–36.
Article22. Locher R, Brandes RP, Vetter W, Barton M. Native LDL induces proliferation of human vascular smooth muscle cells via redox-mediated activation of ERK 1/2 mitogen-activated protein kinases. Hypertension. 2002. 39:645–650.
Article23. Mugabe BE, Yaghini FA, Song CY, Buharalioglu CK, Waters CM, Malik KU. Angiotensin II-induced migration of vascular smooth muscle cells is mediated by p38 mitogen-activated protein kinase-activated c-Src through spleen tyrosine kinase and epidermal growth factor receptor transactivation. J Pharmacol Exp Ther. 2010. 332:116–124.
Article24. Ito T, Ikeda U, Yamamoto K, Shimada K. Regulation of interleukin-8 expression by HMG-CoA reductase inhibitors in human vascular smooth muscle cells. Atherosclerosis. 2002. 165:51–55.
Article25. Geisel J, Jödden V, Obeid R, Knapp JP, Bodis M, Herrmann W. Stimulatory effect of homocysteine on interleukin-8 expression in human endothelial cells. Clin Chem Lab Med. 2003. 41:1045–1048.
Article26. DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992. 90:2123–2129.
Article27. DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993. 268:25568–25576.
Article28. Moreau M, Brocheriou I, Petit L, Ninio E, Chapman MJ, Rouis M. Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation. 1999. 99:420–426.
Article29. Simonini A, Moscucci M, Muller DW, Bates ER, Pagani FD, Burdick MD, et al. IL-8 is an angiogenic factor in human coronary atherectomy tissue. Circulation. 2000. 101:1519–1526.
Article30. Boyle EM Jr, Kovacich JC, Hébert CA, Canty TG Jr, Chi E, Morgan EN, et al. Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 1998. 116:114–121.
Article31. Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009. 84:353–360.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Native low-density lipoprotein-induced superoxide anion contributes to proliferation of human aortic smooth muscle cells
- Multiple Signaling Pathways Contribute to the Thrombin-induced Secretory Phenotype in Vascular Smooth Muscle Cells
- Effect of Oxidezed LDL in Insulin Binding, Internalization and Recycling of Insulin Receptor in Cultured Bovine Aortic Endothelial Cells
- NADPH oxidase activation contributes to native low-density lipoprotein-induced proliferation of human aortic smooth muscle cells
- Signaling Pathway of Lysophosphatidic Acid-Induced Contraction in Feline Esophageal Smooth Muscle Cells